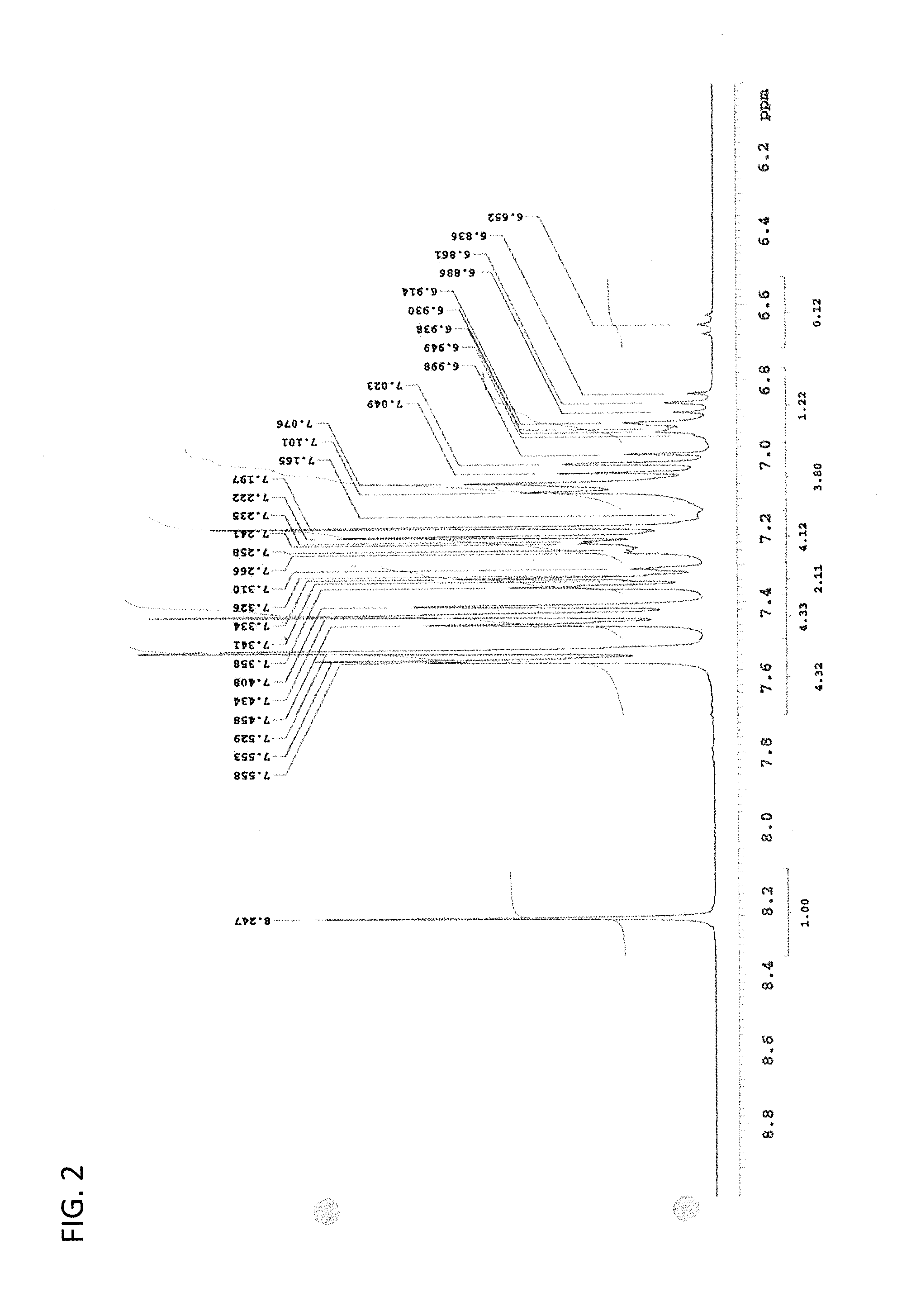
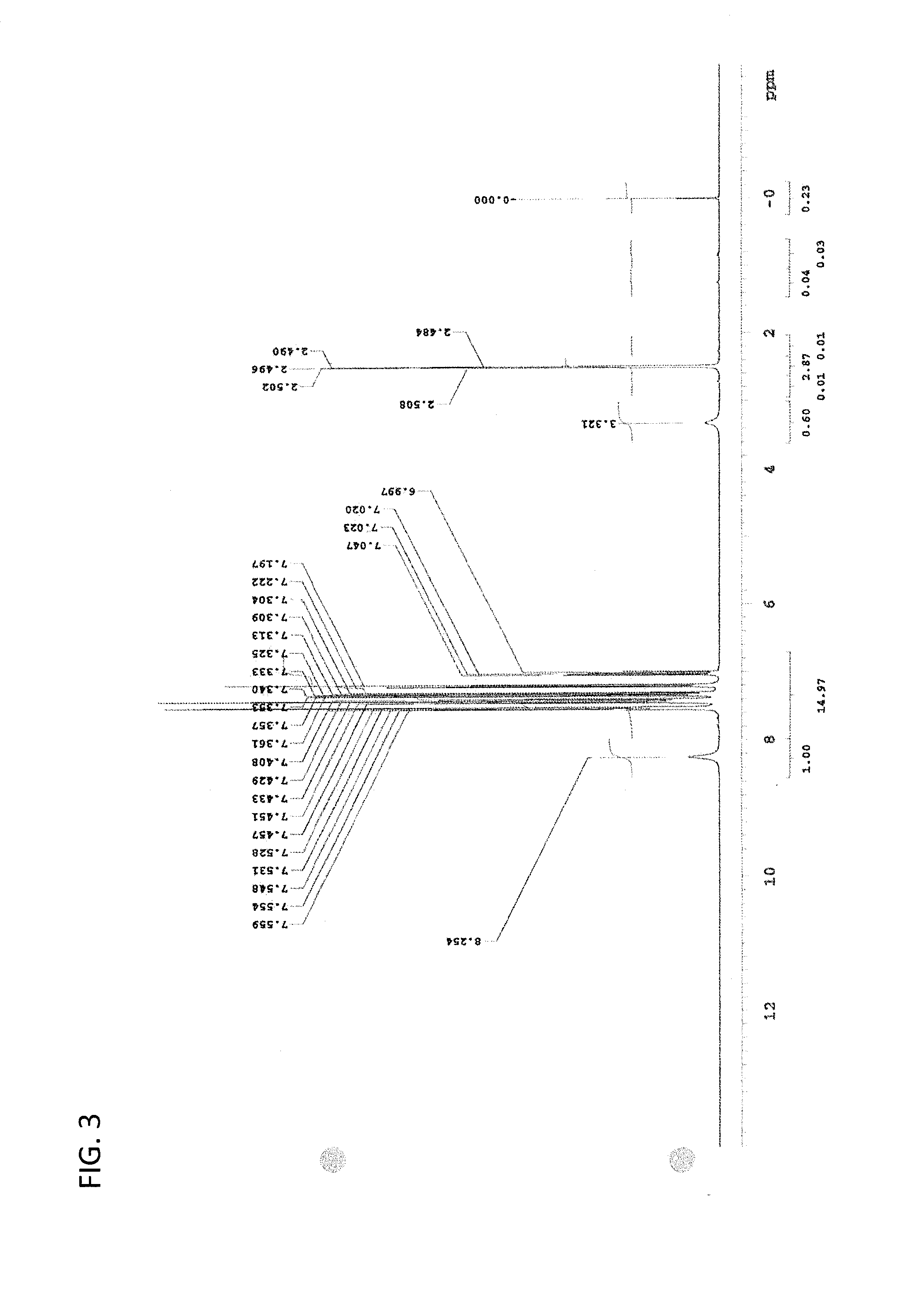
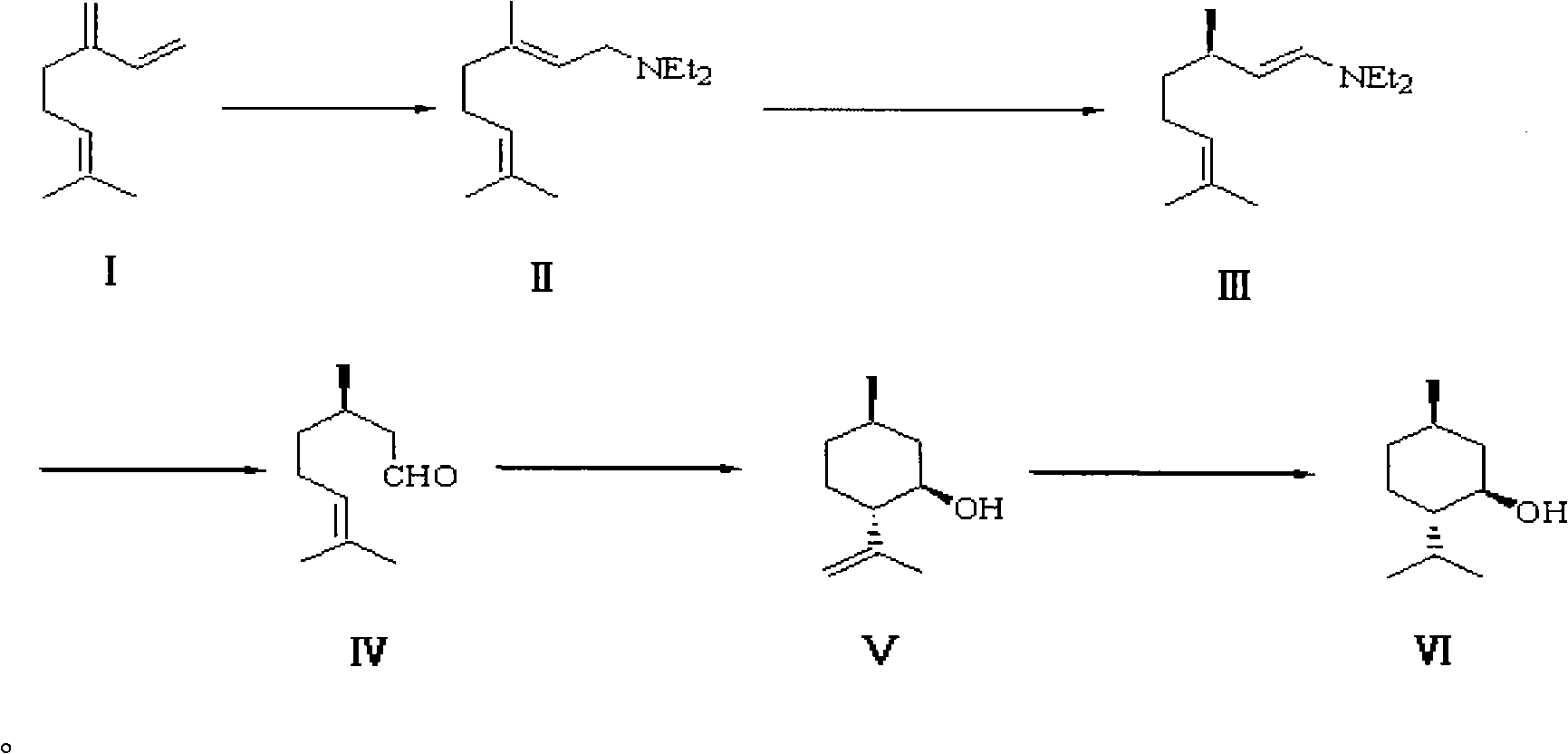
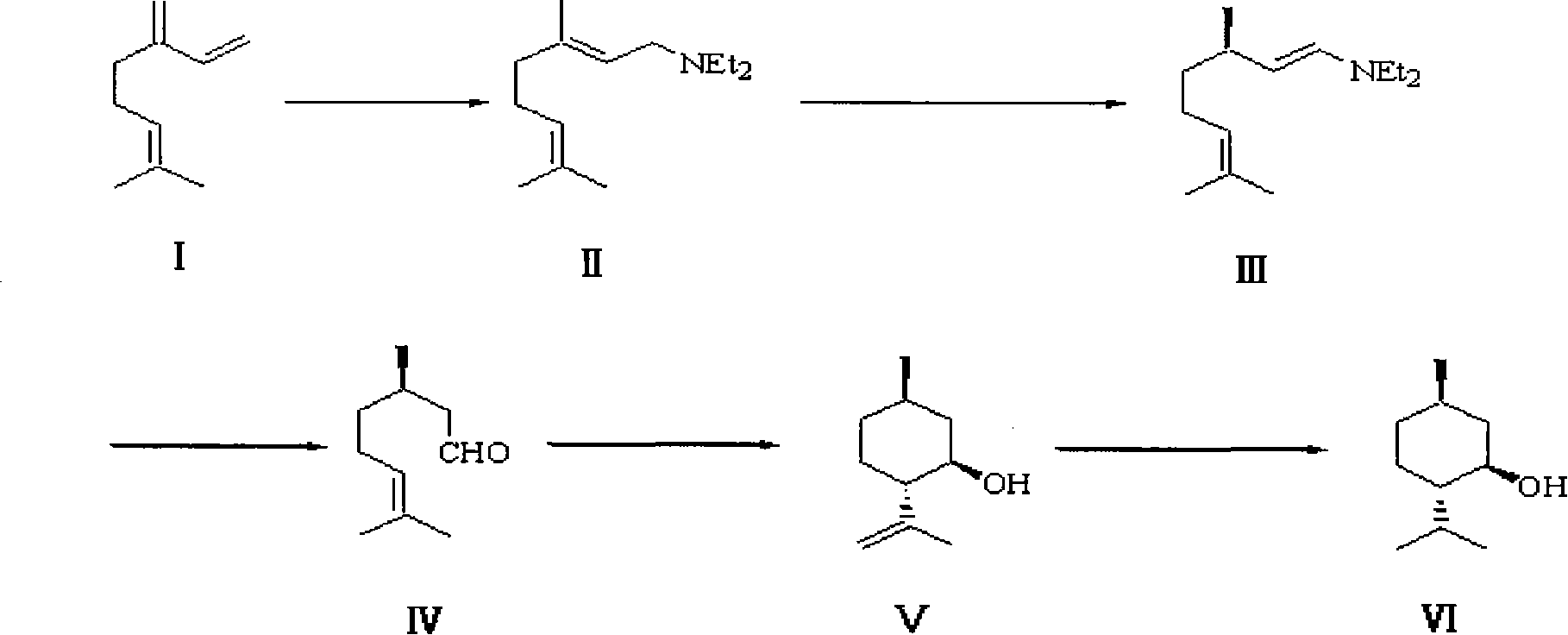
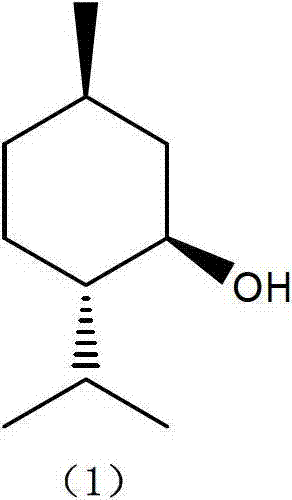
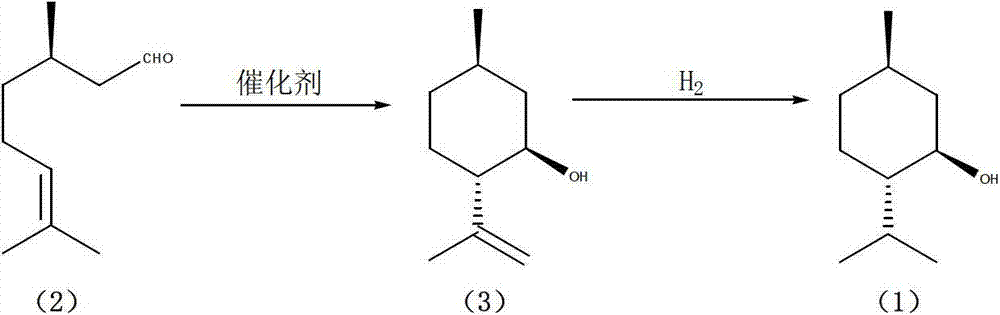
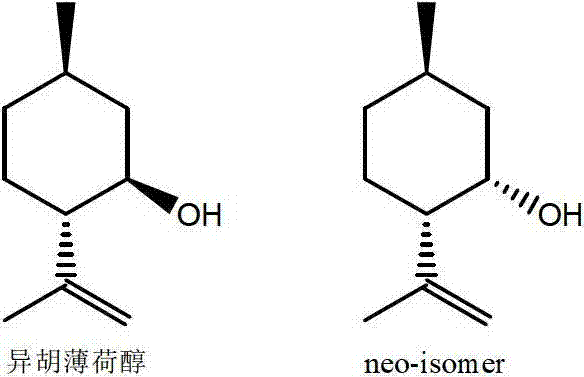
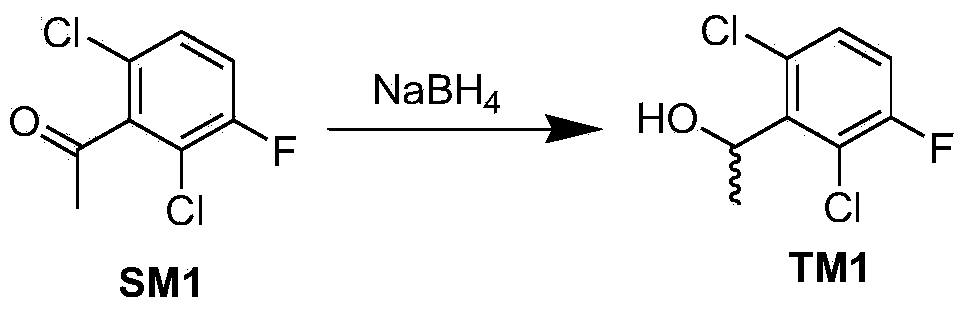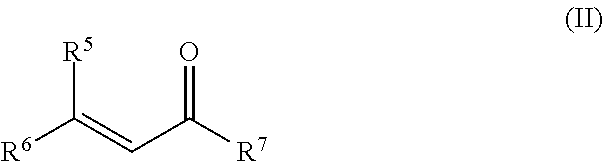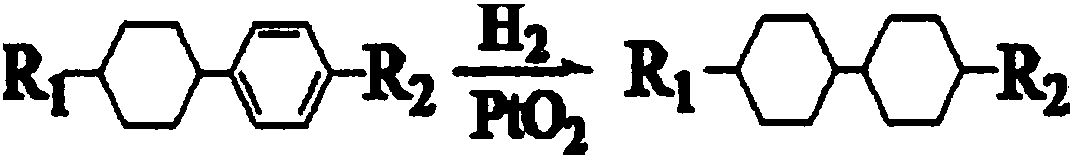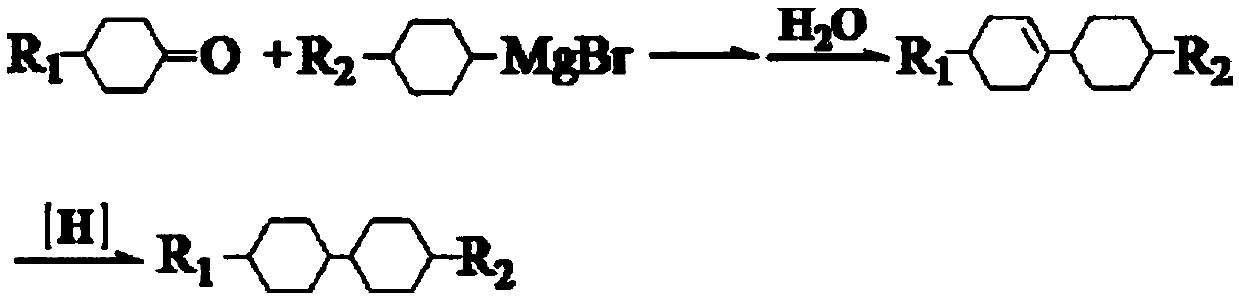Patents
Literature
240results about "Preparation by isomerisation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing two-dimensional metal organic framework material and application thereof
ActiveCN107383386AMild reaction conditionsRapid responsePreparation by isomerisationOrganic-compounds/hydrides/coordination-complexes catalystsMetal-organic frameworkSolvent
The invention discloses a method for preparing a two-dimensional MOFs material, which includes the following steps: organic molecules and a metal organic framework material are mixed under normal temperature, the mixture is then milled or ball-milled, and after washing, centrifuging and drying, the two-dimensional metal organic framework material is obtained. the reaction conditions for the preparation of the two-dimensional MOFs are mild, reaction is fast, yield is high, solvent does not need to be added, moreover, the reaction process is simple, needed equipment is simple, and a ball mill can be used for implementing large-scale production.
Owner:NANJING UNIV OF TECH
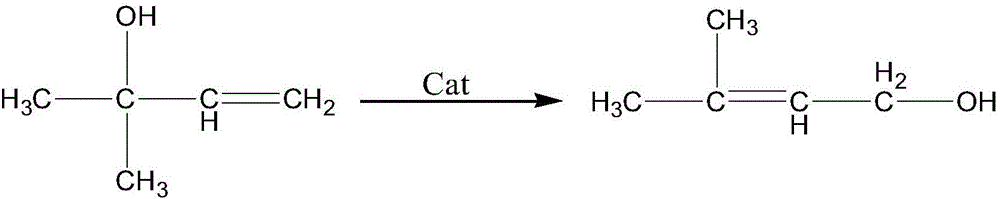
Method for preparing isopentenol from 3-methyl-3-butenol
ActiveCN101544538ALow content of active ingredientsReduce manufacturing costPreparation by isomerisationIsomerizationHydrogen
The invention relates to a method for preparing isopentenol from 3-methyl-3-butenol, which performs isomerization reaction on a raw material of 3-methyl-3-butenol to generate the isopentenol in the presence of a catalyst in an atmosphere containing hydrogen, wherein the reaction pressure is normal pressure, the reaction temperature is between 50 and 90 DEG C, the reaction time is between 25 and 40 minutes, the catalyst uses Al2O3 as a carrier, a load active component is metal Pd, the content of the Pd in the catalyst in percentage by weight is between 0.1 and 2.0 percent, the particle size of the catalyst is between 80 and 200 meshes, and the weight ratio of the raw material to the catalyst is 30-100:1. Compared with the prior art, the method has obvious improvement, the catalyst is simpler, and the content of the active component is lower, thus the preparation is easier and the cost is lower; the reaction condition is mild; and on the premise of ensuring the selectivity and the product yield of a perfect product, the reaction time is greatly shortened. The reduction of the preparation cost of the catalyst and shortening of the reaction time finally reduce the overall production cost greatly.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Method for manufacturing optically active menthol
InactiveUS20130253228A1Easy to operateHigh selectivityOxygen-containing compound preparationPreparation by isomerisationMentholAsymmetric hydrogenation
An object of the present invention is to provide a method for manufacturing an optically active menthol having fewer steps, which generates less environmentally polluting waste because a catalytic reaction is involved in all of the steps, and is capable of saving a production cost. The present invention relates to a method for manufacturing an optically active menthol, including the following steps: A-1) asymmetrically hydrogenating at least one of geranial and neral to thereby obtain an optically active citronellal, B-1) conducting a ring-closure reaction of the optically active citronellal in the presence of an acid catalyst to thereby obtain an optically active isopulegol, and C-1) hydrogenating the optically active isopulegol to thereby obtain an optically active menthol.
Owner:TAKASAGO INTERNATIONAL CORPORATION
Method for synthesizing L-menthol
InactiveCN101602651AWide variety of sourcesLow pricePreparation by isomerisationPreparation by hydrogenationIsomerizationOrganic synthesis
The invention belongs to the technical field of organic synthesis, in particular to a method for synthesizing L-menthol. The method for synthesizing L-menthol takes myrcene as an initial raw material and comprises the following steps: amination reaction: diethylamine and myrcene carry out amination reaction to obtain geranylamine; isomerization reaction: the geranylamine carries out isomerization reaction under the catalytic action of chiral catalyst to obtain intermediate enamine; hydrolysis reaction: the enamine carries out hydrolysis reaction in an acidic medium to produce intermediate D-citronellal; cyclization reaction: the D-citronellal generates L-isopulegol by lewis acid catalyst and organic solvent; and hydrogenation reaction: the L-isopulegol is hydrogenated to obtain L-menthol. The method for synthesizing L-menthol has the yield of more than 90 percent, the GC purity of higher than 99.0 percent and the specific rotation of -49 degrees to -50 degrees, the raw material myrcene has wide sources and lower cost, and the whole method has simple technology, good repeatability and high yield and is quite suitable for large-scale industrialized production.
Owner:SHANGHAI WANXIANG FLAVORS & FRAGRANCES
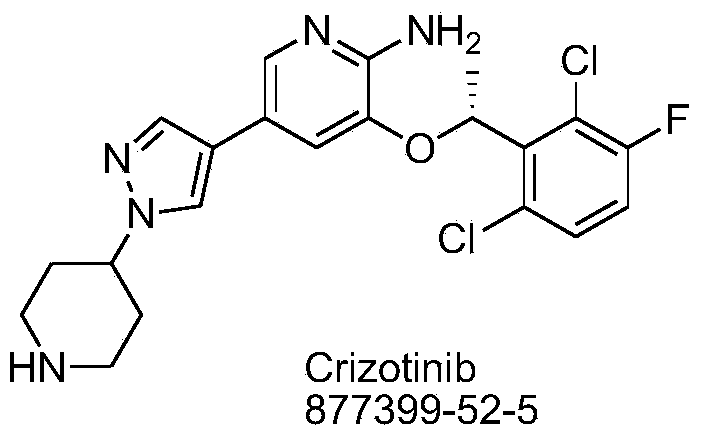
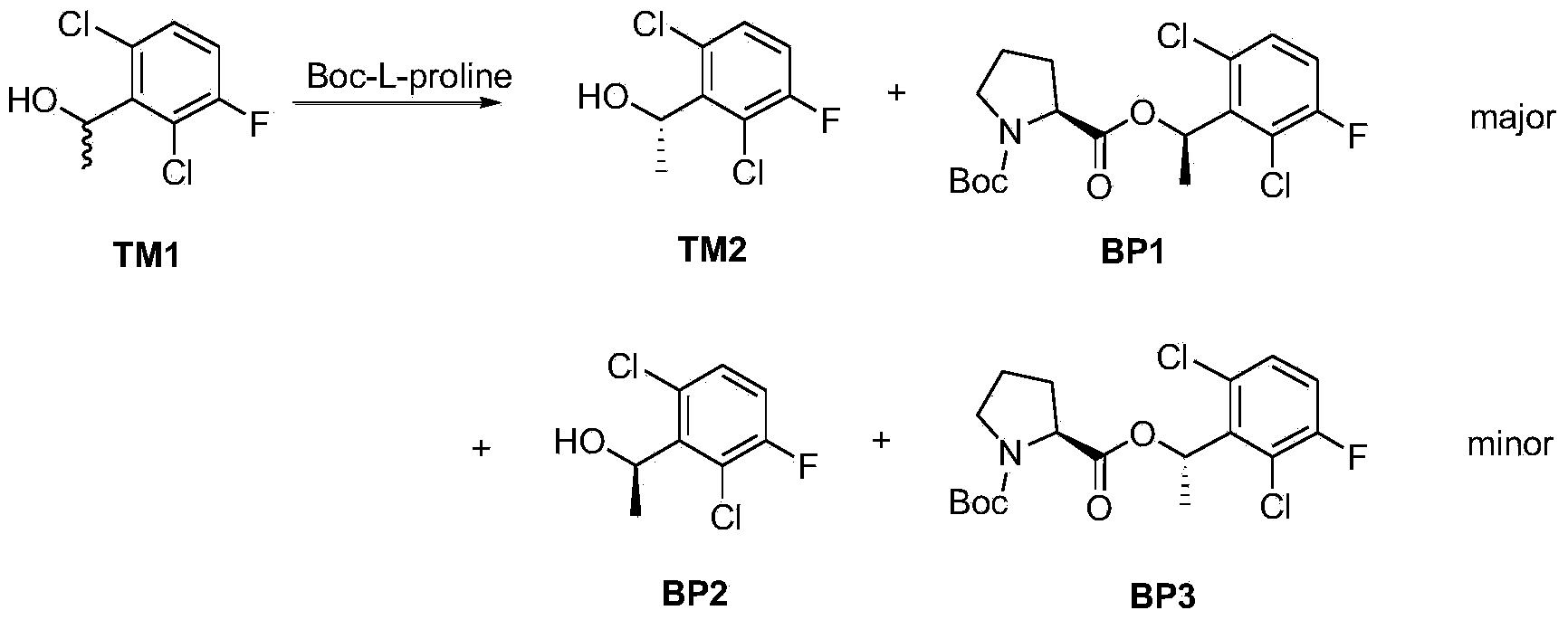
Synthetic process method for novel antineoplastic molecular targeted drug of crizotinib
ActiveCN103664896AReduce processing stepsEmission reductionPreparation by isomerisationOrganic compound preparationP-Toluenesulfonic acidHydrolysis
The invention provides a synthetic process method for a novel antineoplastic molecular targeted drug of crizotinib, and relates to the resolution process optimization of chiral isomers of a crizotinib precursor and the recycling of by-products. The method adopts a catalyzing resolution method that Boc-L-proline, namely, N-(tert-butoxycarbonyl)-L-proline) is combined with a catalyst of p-toluenesulfonic acid and a condensating agent of 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride to split 1-(2,6-dichloro-3-fluorophenyl)ethanol racemate into S-type alcohol and R-type alcohol, and a resolution by-product mixture is subjected to hydrolysis and configuration transition to obtain the (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol; the total yield is improved to 76% from 30%, the time is shortened, pollution is reduced, and application to industrialized production is easy.
Owner:济南良福精合医药科技有限公司
Process for catalytically synthesizing isosorbierte by strong acid ion exchanging resin
InactiveCN1425637AEliminate carbonizationNo pollution in the processPreparation by isomerisationAlcoholStrong acids
The synthesis process includes adding dewaternig agent solution in the amount of 10-50 wt% of industrial water solution of sorbierte to industrial water solution of sorbierte through stirring and heating to 100-120 deg.c to dewater sorbierte; adding strong acid ion exchanging resin as catalyst in the amount of 6-15 wt% of industrial water solution of sorbierte through stirring and heating to 104-110 deg.c to dewater until finishing the reaction; adding water to dilute and filtering out strong acid ion exchanging resin and separating dewatering agent; distilling to obtain course product; and re-crystallizing in mixed ethanol and ether solvent to obtain isosorbierte product.
Owner:QUFU NORMAL UNIV
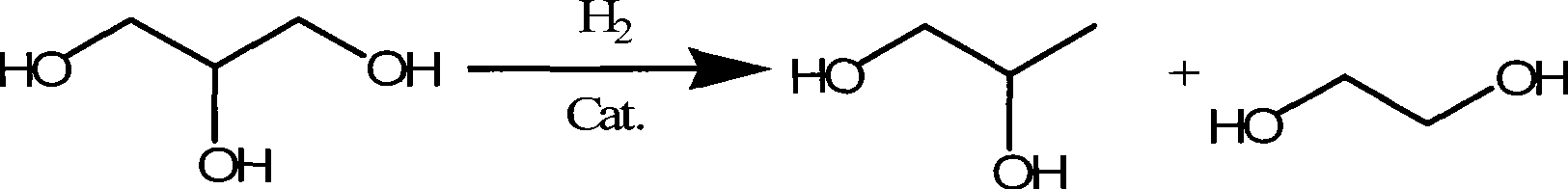
Method for hydrocracking glycyl alcohol
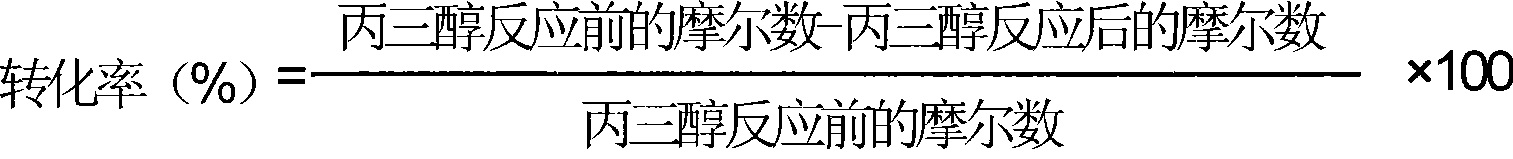
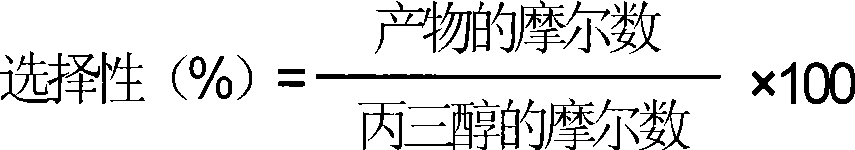
InactiveCN101372444ALow costImprove practicalityPreparation by isomerisationPreparation by OH group eliminationAlcoholPropylene glycol
The invention relates to a catalyst for glycerin hydrocracking. The catalyst is a supported amorphous catalyst with two functions, high catalytic activity, mild reaction conditions, pollution-free, and clean and environment-friendly reaction process. Under the catalysis of the catalyst, the glycerin is hydrocracked to generate ethylene glycol and 1, 2-propylene glycol with the conversion of more than 95%; and the overall selectivity of the ethylene glycol and the 1, 2-propylene glycol is more than 85%.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
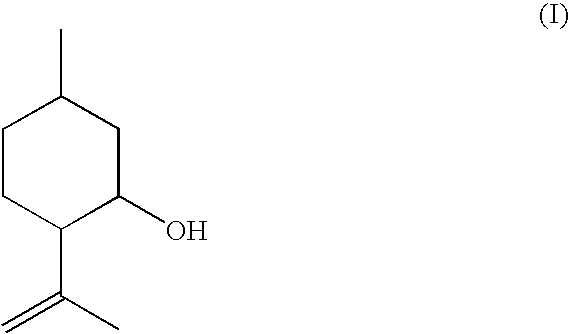
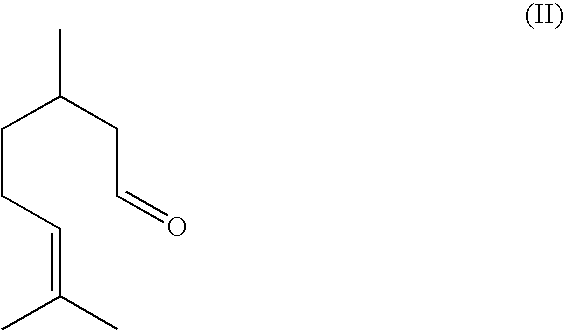
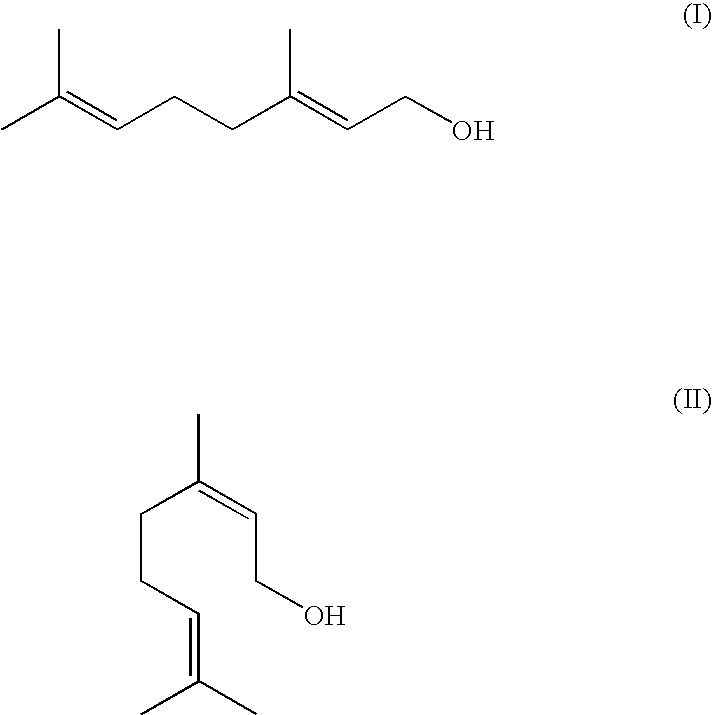
Method For The Production Of Isopulegol
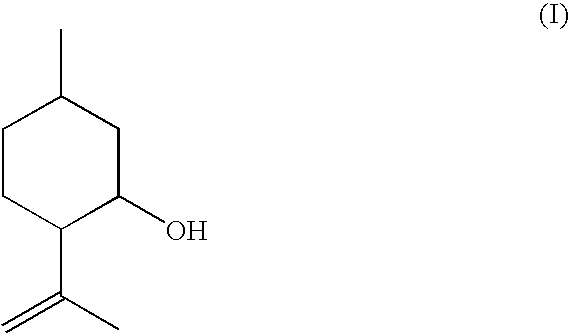
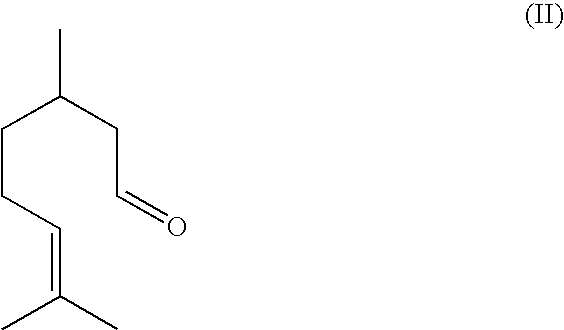
ActiveUS20080207957A1Suppress formationOxygen-containing compound preparationPreparation by isomerisationVinyl etherKetone
The present invention relates to a process for the preparation of isopulegol of formula (I):comprising the cyclization of citronellal of formula (II):in the presence of a tris(aryloxy)aluminum catalyst, wherein the cyclization is carried out in the presence ofI. at least one acid and / orII. at least one compound selected from the group comprising carboxylic anhydrides, aldehydes, ketones and vinyl ethers.
Owner:BASF AG
Method for synthesizing optically active carbonyl compounds
ActiveUS20180057437A1Improve stabilityExtended service lifePreparation by isomerisationOrganic compound preparationArylHydrogen
The present invention relates to a process for the preparation of an optically active carbonyl compound by asymmetric hydrogenation of a prochiral α,β-unsaturated carbonyl compound with hydrogen in the presence of at least one optically active transition metal catalyst that is soluble in the reaction mixture and which has rhodium as catalytically active transition metal and a chiral, bidentate bisphosphine ligand, wherein the reaction mixture during the hydrogenation of the prochiral α,β-unsaturated carbonyl compound additionally comprises at least one compound of the general formula (I):in whichR1, R2: are identical or different and are C6- to C10-aryl which is unsubstituted or carries one or more, e.g. 1, 2, 3, 4 or 5, substituents which are selected from C1- to C6-alkyl, C3- to C6-cycloalkyl, C6- to C10-aryl, C1- to C6-alkoxy and amino;Z is a group CHR3R4 or aryl which is unsubstituted or carries one or more, e.g. 1, 2, 3, 4 or 5, substituents which are selected from C1- to C6-alkyl, C3- to C6-cycloalkyl, C6- to C10-aryl, C1- to C6-alkoxy and amino, wherein R3 and R4 are as defined in the claims and the description.
Owner:BASF AG
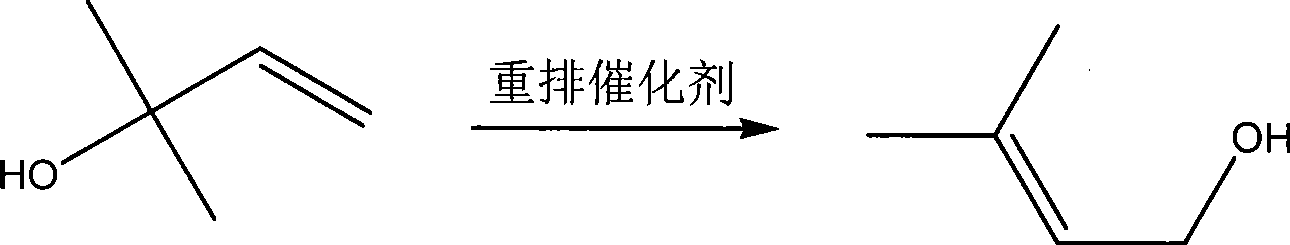
Method for continuously preparing 3-methyl-2-butenol
InactiveCN101381283AOperations that reduce recyclingRaw materials are easy to getPreparation by isomerisationButeneSolvent
The invention discloses a method for preparing intermediate 3-methyl-2-butenol. The prior method has the defects of rigorous conversion reaction condition, low conversion rate, difficult separation, and high technical requirement. The method has the following steps of: putting 2-methyl-3-butene-2-hydrin and rearrangement catalyst into a reaction kettle, and heating the 2-methyl-3-butene-2-hydrin and rearrangement catalyst to undergo a catalytical rearrangement reaction to obtain the mixture of the 2-methyl-3-butene-2-hydrin and the 3-methyl-2-butenol; separating the mixture through a rectification tower, recovering the 2-methyl-3-butene-2-hydrin at the top of the rectification tower and returning the 2-methyl-3-butene-2-hydrin to the reaction kettle for feeding, obtaining the crude product of the 3-methyl-2-butenol through discharging from a lateral line of the rectification tower, and continuously replenishing new 2-methyl-3-butene-2-hydrin into the reaction kettle. The method has the advantages of no use of solvent, mild reaction condition and easy control, and realizes the continuous production based on the continuous feeding and discharging.
Owner:ZHEJIANG NHU CO LTD +1
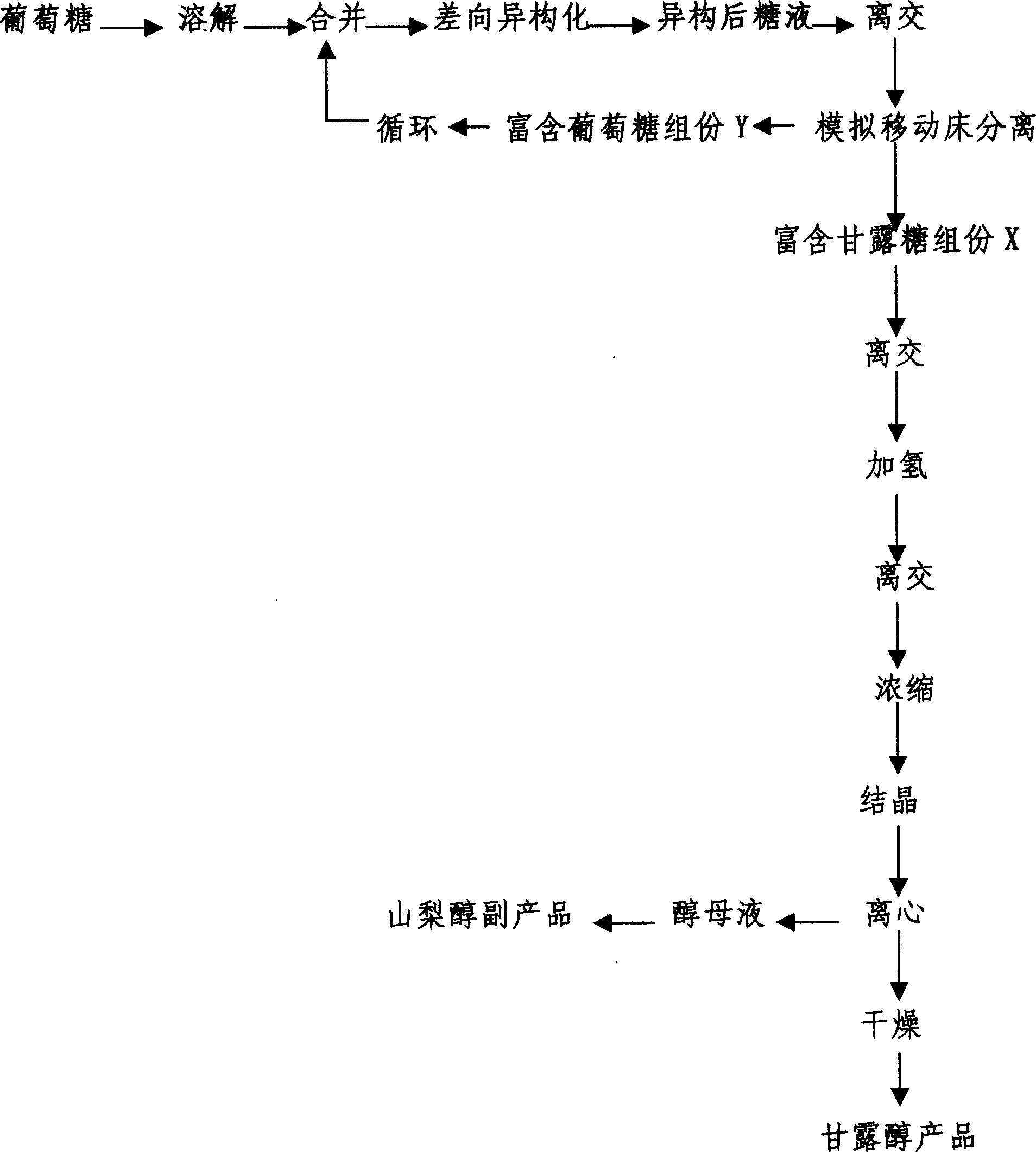
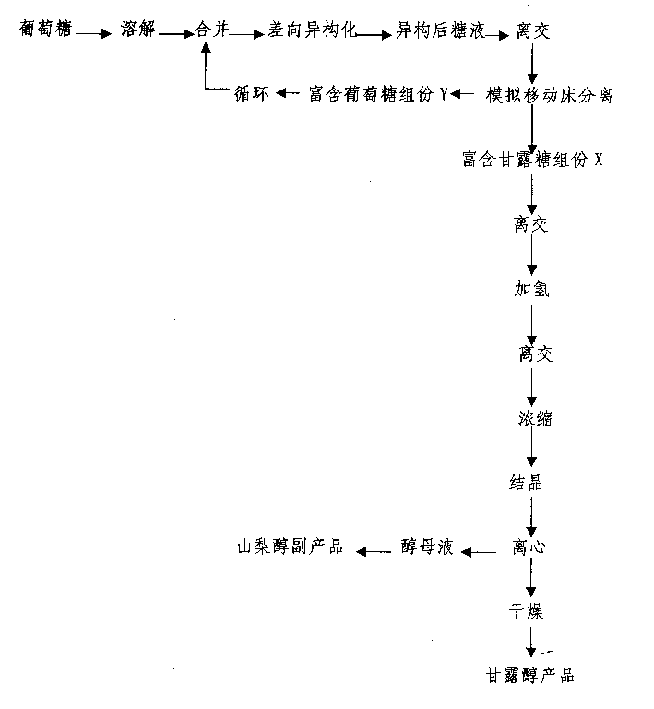
High-yield manna sugar preparation process
The invention is a high-yield mannitol preparing method using glucose as raw material, on acidic condition, making the glucose through epimerization reaction to obtain the mixture mannitose-glucose, separate mannitose-glucose by simulated moving bed at 40-85 deg.C, using calcium cation exchange resin as sorbent and water as eluent to continuously obtain glucose-rich component and mannitose-rich component, where the former can repeatedly make epimerization reaction, the latter can generate manitol when added with hydrogen and the mannitol content in the mixed alcohol added with hydrogen is up to above 75%, then crystallizing, centrifuging and drying to obtain the mannitol product, where mannitol productivity is 70% of dry glucose charging capacity.
Owner:南宁市化工研究设计院
Method for preparing 3-methyl-2-butenol
ActiveCN107141197AAvoid generatingHigh activityPreparation by isomerisationOrganic compound preparationIsomerizationOrganic base
The invention provides a method for preparing 3-methyl-2-butenol (isoprenol) through isomerization of 3-methyl-3-butenol. A catalyst reaction system contains 1) a carbonyl iron compound, 2) an organic base, and 3) an epoxy group ligand. A solvent with an ether structure is added into an isomerization reaction, so that the activity of a catalyst can be improved, and the product selectivity is improved. During catalysis and separation, a mixed gas of carbon monoxide (CO) and nitrogen (N2) is introduced, wherein the volume content of the CO is 100-100000ppm. The catalyst and a raw material namely the 3-methyl-3-butenol are separated from a product namely isoprenol through rectification, and the catalyst does not need to be additionally separated. The catalyst keeps high activity and long life operation.
Owner:WANHUA CHEM GRP CO LTD
Process for synthesizing geraniol/nerol from linalool
The method for synthesizing geraniol and neol from linalool is characterized by that in the linalool the transition metal or its oxide can be added as catalyst, the boric acid polyglycol ester or boric acid polylol ester whose molecular weight is 5000-10000 can be added as adjuvant, under the condition of that its pressure is 0.01-1.0 MPa and temp. is 50-250 deg.C they are reacted for 3-12 hr. inwhich the dose of the catalyst is 0.1-1.0% of mass percentage ratio of linalool. It can greatly reduce raw material cost, the catalyst and adjuvant can be continuously and repeatedly used.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Piperitol isomerization catalyst and method for continuously obtaining L, D-piperitol
ActiveCN103143363AEasy to separateEasy to manufacturePreparation by isomerisationPreparation by hydrogenationIsomerizationMetal catalyst
The invention relates to a piperitol isomerization catalyst and a method for continuously obtaining L, D-piperitol. The catalyst is a binary metal catalyst and comprises the following components by weight percentage: 8-10% of nickel, 3-5% of copper, and the balance of carrier materials. The isomerization catalyst comprises the components of nickel and copper and can realize the isomerization of piperitol at low temperature and low pressure, the yield of the L, D-piperitol is high and the operation steps are simple.
Owner:SANMING UNIV
Method for preparing L-menthol
InactiveCN103086845AAvoid purification difficultiesAvoid Yield ProblemsPreparation by isomerisationMolecular sieve catalystsZinc bromideMolecular sieve
The invention discloses a method for preparing L-menthol, which comprises the following steps:(1) reacting d-citronellal in a solvent in the presence of a catalyst, and collecting isopulegol from the reaction product, wherein the catalyst is a zinc-bromide-modified NaY molecular sieve; and carrying out high-pressure hydrogenation on the obtained isopulegol to obtain the target product L-menthol. The obtained isopulegol can be subjected to high-pressure hydrogenation to obtain the target product L-menthol without purification. The invention has the characteristics of mild reaction conditions, high stereoselectivity, high yield and the like, and is simple to operate; and the catalyst is simple to recover, and can be used repeatedly. The method disclosed by the invention avoids the problems of difficulty in product purification, low yield and the like in the traditional synthesis technique of the compounds, and greatly lowers the production cost.
Owner:上海统益生物科技有限公司
Method For The Production Of Menthol
ActiveUS20080139852A1Easy to getOxygen-containing compound preparationPreparation by isomerisationMentholEnantio selectivity
Processes comprising: (a) enantioselectively hydrogenating a starting material comprising a component selected from geraniol, nerol and mixtures thereof to form optically active citronellol; (b) converting the optically active citronellol to optically active citronellal; (c) cyclizing the optically active citronellal to form a mixture comprising optically active isopulegol; and (d) subjecting the mixture to further processing comprising: (i) separating the optically active isopulegol from the mixture and hydrogenating the separated optically active isopulegol to form optically active menthol; or (ii) hydrogenating the optically active isopulegol in the mixture to form optically active menthol and separating the optically active menthol from the mixture.
Owner:BASF AG
Method for synthesizing prenol by composite catalyst
ActiveCN102675048ARaw materials are easy to getLow costPreparation by isomerisationButenePtru catalyst
The invention discloses a method for synthesizing prenol by composite catalyst. Generally, rare metals high in price and small in number of application times are used in the existing synthetic methods, and accordingly product cost is increased in industrial production, and product competitiveness is lowered. The method for synthesizing prenol by composite catalyst is characterized by using 3-methl-3-butene-1-ol as raw material, using composite catalyst composed of raney nickel and solid super acid, hydrogenating, and subjecting the 3-methl-3-butene-1-ol double-bond isomerization to synthesize prenol. The composite catalyst is easy to obtain, low in cost, large in number of application times, high in conversion rate and selectivity, low in reaction temperature and the like. Reaction temperature is low, and the method is low in energy consumption and meets the technological requirements on energy conservation and consumption reduction.
Owner:SHANDONG NHU PHARMA +1
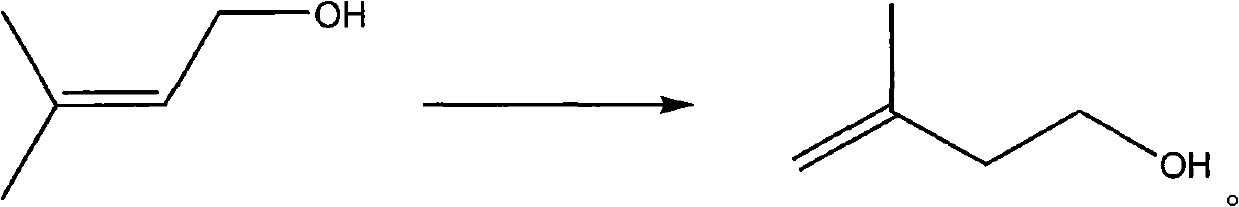
Method for preparing 3-methyl-3-butene-1-alcohol
InactiveCN101993353AImprove conversion rateReduce energy consumptionPreparation by isomerisationOrganic compound preparationButeneAlcohol
The invention discloses a method for preparing a 3-methyl-3-butene-1-alcohol. The method comprises the following step of: performing catalytic isomerization reaction on isoamylene alcohol under the actions of Pd / Al2O3 catalyst and hydrogen to obtain the 3-methyl-3-butene-1-alcohol. The preparation method can greatly reduce the energy consumption, and has the characteristics of low investment and low production cost; and the selectivity of a target product can reach 100 percent, and the conversion rate of the isoamylene alcohol and the purity of the product can reach over 98.5 percent.
Owner:SHANGHAI CHENGJIE IND
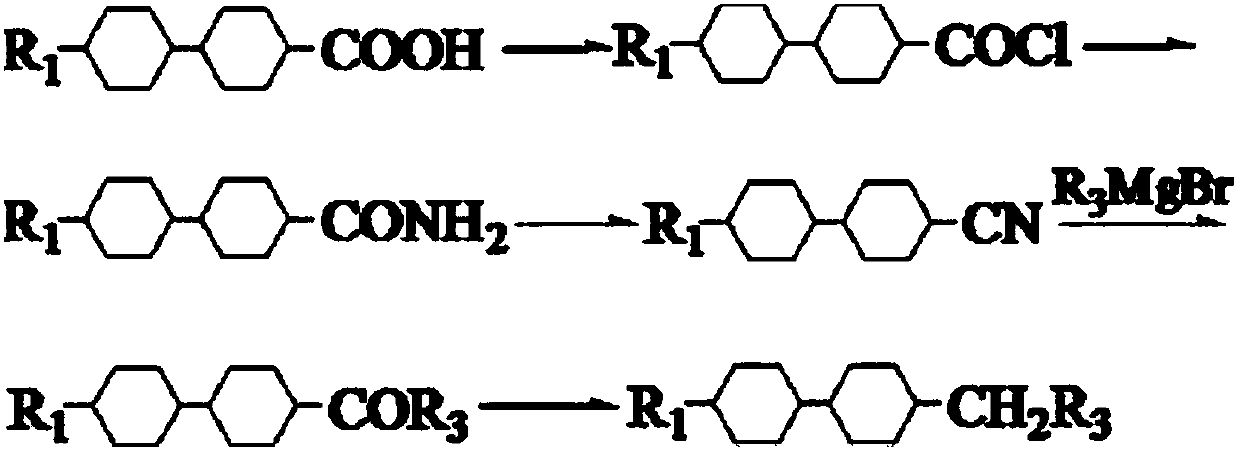
New preparation method of trans-trans dicyclohexyl alkane liquid crystal compounds
InactiveCN109534948ARealize green chemical industryHigh yieldLiquid crystal compositionsPreparation by isomerisationAlkaneChemical industry
The invention discloses a preparation method of trans-trans dicyclohexyl alkane liquid crystal compounds. According to the preparation method, 1-(4-(4-alkylcyclohexyl)phenyl)alkyl-1-one is used as a raw material; a benzene ring is converted into a cyclohexyl ring by means of a catalytic hydrogenation reaction; transposition is performed under the alkaline condition, so that 1-(4-(4-alkylcyclohexyl)cyclohexyl)alkyl-1-ol is obtained; the trans-trans dicyclohexyl alkane liquid crystal compounds are finally obtained by carrying out an oxidation reaction and a reduction reaction. The preparation method provided by the invention is unique in synthetic route, not only realizes green chemical industry, but also enables the product yield to be greatly increased, improves the product quality, and reduces the cost.
Owner:YANTAI DERUN LIQUID CRYSTAL MATERIALS
Isomerization synthesis of 3-methyl-2-buten-1-ol using water-containing methyl butenol
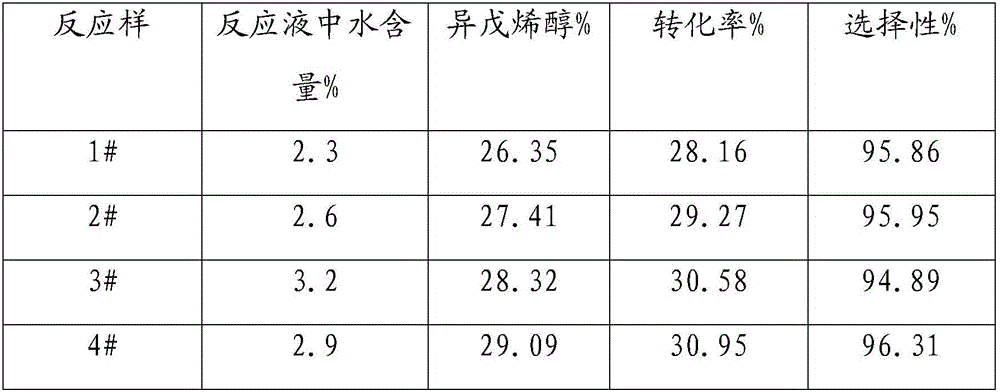
ActiveCN105967978AHigh reactivityHigh selectivityPreparation by isomerisationIsomerization3-methyl-2-buten-1-ol
The invention belongs to the field of chemical engineering and particularly relates to isomerization synthesis of 3-methyl-2-buten-1-ol using methyl butenol in a water-containing condition. In the invention, the methyl butenol is adopted as a raw material for isomerization synthesis of 3-methyl-2-buten-1-ol; in the reaction materials, the water content is 0.5-6.5%; the catalyst is vanadium oxide or vanadium-containing metal salt; the catalyst concentration is 0.1-5.0%, the reaction temperature is 120-190 DEG C, and the reaction time is 20-180min; the purity of the obtained 3-methyl-2-buten-1-ol product is higher than or equal to 98%; and the 3-methyl-2-buten-1-ol selectivity in the reaction process can be controlled at 95% or above. The invention has the following advantages: the adopted catalyst does not need to be prepared in advance and is relatively cheap; the raw material methyl butenol can be adopted for isomerization synthesis of 3-methyl-2-buten-1-ol in a water-containing condition; and the reaction activity and selectivity are relatively high.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
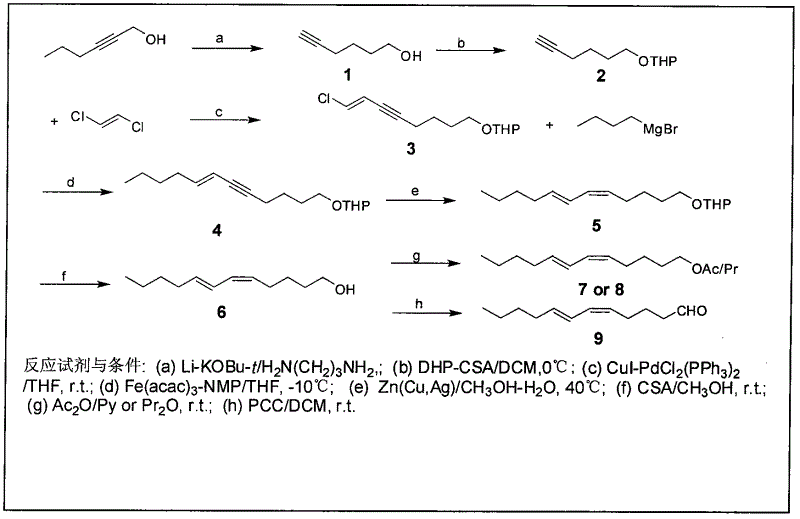
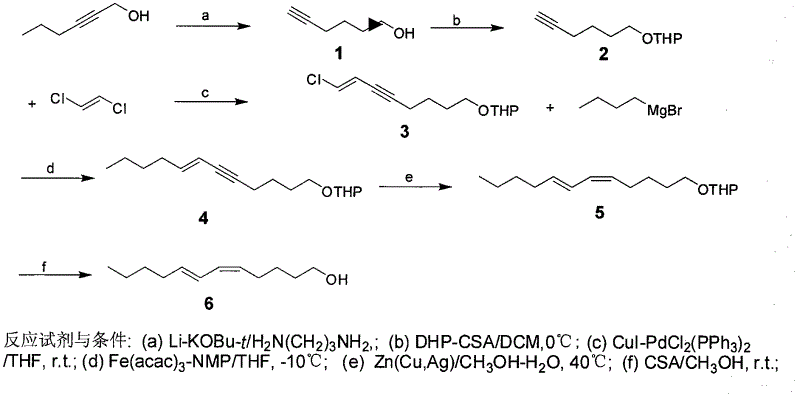
Process for synthesizing sex pheromone of pine caterpillar
ActiveCN102613177AEasy to operateSynthetic raw materials are readily availableBiocidePreparation by isomerisationGrignard reagentIodide
The invention discloses a process for synthesizing sex pheromone of pine caterpillar, which employs 2-hexyne-1-alcohol as an initial raw material, three-bond positional transference is carried out under the effect of lithium and propane diamine to obtain 5-hexyne-1-alcohol; under acidic condition, 5-hexyne-1-alcohol is reacted with dihydropyran to obtain 1-THP-5-hexyne-1-alcohol protected by THP on hydroxyl, Under co-catalysis of metal palladium and cuprous iodide, 1-THP-5-hexyne-1-alcohol and trans-dichloroethylene are subjected to coupling reaction to generate conjugate enyne(7E)-1-THP-8-chlorine-5-alkyne-7-alkene-1-octanol; under the catalysis of metallic iron, (7E)-1-THP-8-chlorine-5-alkyne-7-alkene-1-octanol and a n-Butyl bromide grignard reagent are further subjected to coupling reaction to obtain (7E)-1-THP-5-alkyne-7-alkene-1-dodecanol, under the catalytic reduction of metal zinc, (5Z, 7E)-1-THP-dodecanol dienol; under the camphor sulfonic acid condition, (5Z, 7E)-1-THP-dodecanol dienol removes the THP protective group to obtain the final target product (5Z, 7E)-dodecanol dienol. The method of the invention has the advantages of easily available synthesis raw materials, low cost, mild reaction condition, easy operation, high yield and good stereoselectivity.
Owner:WENZHOU MEDICAL UNIV +1
Heterogeneous catalyst and preparation method thereof as well as method for preparing 3-methyl-2-butene-1-ol in presence of catalyst
ActiveCN103861633AHigh selectivityImprove stabilityPreparation by isomerisationMolecular sieve catalystsLanthanide2-Butene
The invention provides a heterogeneous catalyst and a preparation method thereof as well as a method for preparing 3-methyl-2-butene-1-ol in the presence of the catalyst. The heterogeneous catalyst comprises a carrier and three components on the carrier, wherein the first component is selected from one or two or several in palladium, gold, platinum, nickel, chromium and molybdenum; the second component is selected from one or two or several in elements of groups IIIA, IVA and VA; the third component is selected from one or two or several in lanthanide elements. The preparation method comprises the step of performing a reactive distillation process on 3-methyl-3-butene-1-ol to prepare the 3-methyl-2-butene-1-ol in the presence of the heterogeneous catalyst. According to the method provided by the invention, the yield and selectivity of the 3-methyl-2-butene-1-ol can be obviously improved, and the stability of the catalyst is improved, so that the production cost is reduced.
Owner:WANHUA CHEM GRP CO LTD +1
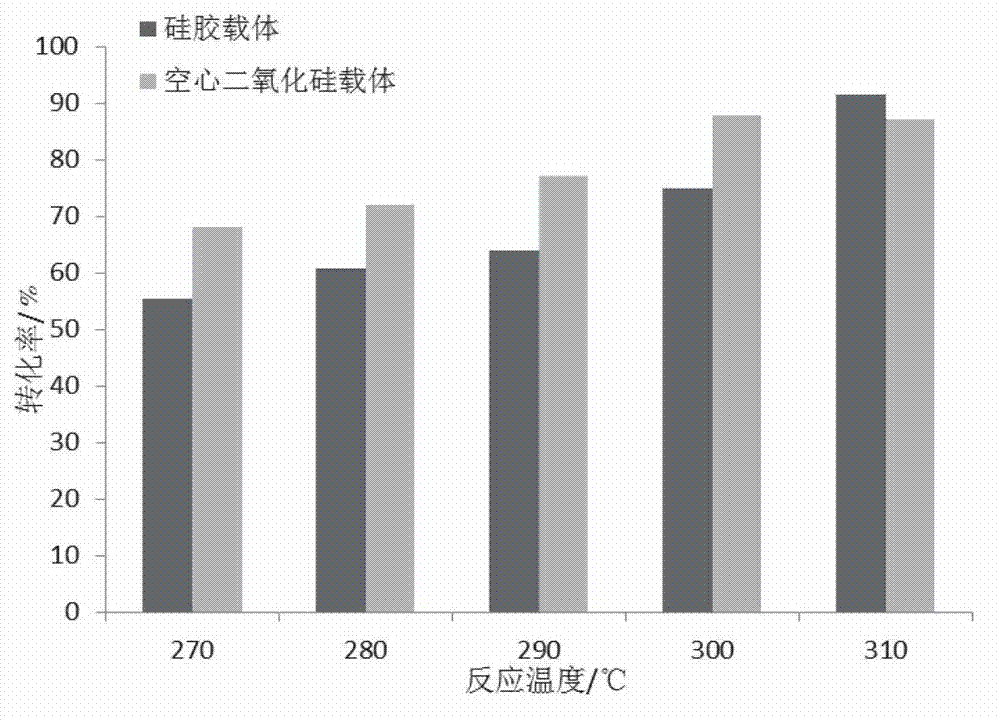
Silica-supported lithium phosphate catalyst in eggshell shape, and preparation method and application thereof
InactiveCN103586054ACatalytic conversion rate does not decreaseImprove conversion ratePreparation by isomerisationPhysical/chemical process catalystsEpoxyAlkane
The invention discloses a silica-supported lithium phosphate catalyst and a preparation method thereof. The catalyst, with hollow nano silica microsphere as a carrier and loaded with basic lithium phosphate, can be used for gas phase isomerization of epoxy propane. The preparation method is as below: preparing a nano hollow spherical silica microsphere with high specific area by using functionalized polystyrene as a template; preparing basic lithium phosphate by using a water solution containing lithium and alkali metal ions and a water solution containing phosphate ions; and then loading the lithium phosphate onto the silica microsphere to prepare the supported catalyst. The obtained silica supported lithium phosphate catalyst can be used in reaction for isomerization of epoxy alkane into corresponding allylic alcohol, and has higher conversion rate and selectivity at low temperature.
Owner:NANJING UNIV OF SCI & TECH
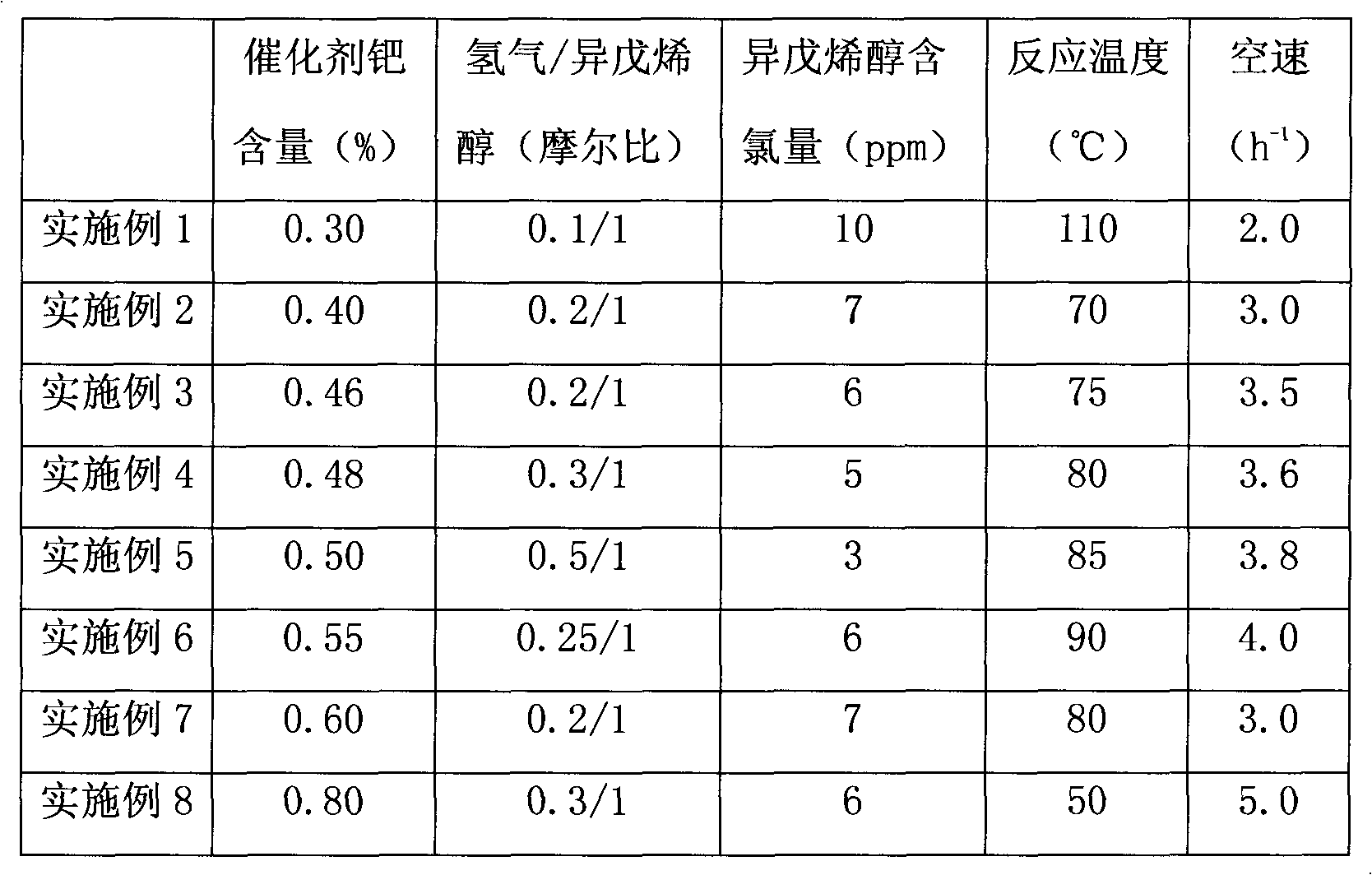
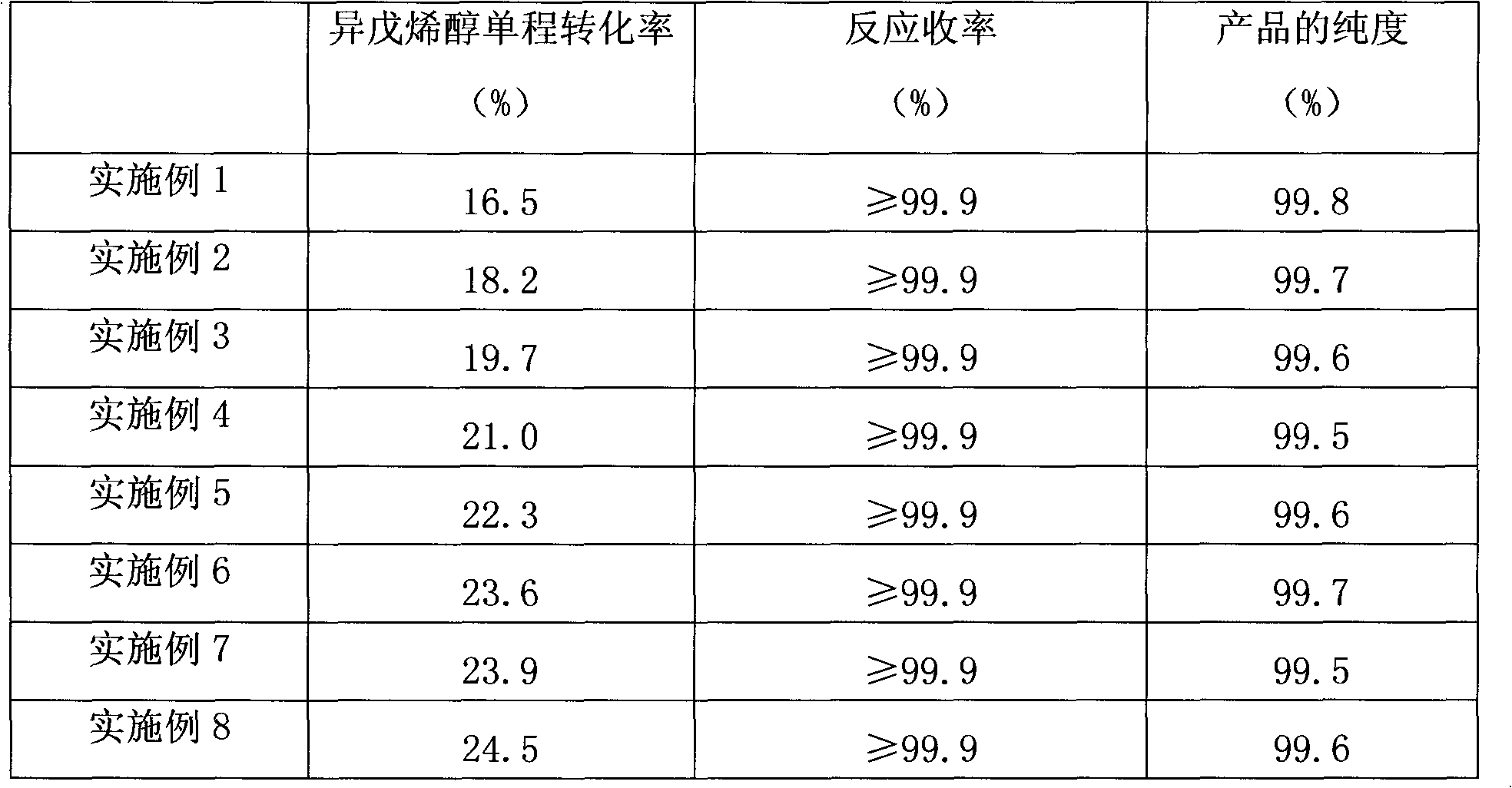
3-methyl-3-butenyl-1-alcohol production method
InactiveCN102367220AOvercome the problem of complex production processPreparation by isomerisationHydrogenIsomerization
The invention discloses a 3-methyl-3-butenyl-1-alcohol production method. The 3-methyl-3-butenyl-1-alcohol production method is characterized in that isoprenol as a raw material undergoes an isomerization reaction in a fixed bed reactor, wherein an isomerization catalyst is Pd / SiO2; Pd content is in a range of 0.3 to 0.8%; a reaction temperature is in a range of 50 to 110 DEG C; reaction pressure is ordinary pressure; a molar ratio of hydrogen to isoprenol is in a range of 0.1 to 0.5; a mass space velocity is in a range of 2 to 5h<-1>; and organic chlorine content of isoprenol is in a range of 3 to 10ppm. Compared with the existing 3-methyl-3-butenyl-1-alcohol production method, the 3-methyl-3-butenyl-1-alcohol production method provided by the invention solves the problems that the existing 3-methyl-3-butenyl-1-alcohol production method has a high raw material consumption rate and a high production cost and can cause severe environmental pollution, and has the advantages of simple processes, abundant raw material sources, low production cost, high reaction yield and low pollution on the environment.
Owner:赵明江
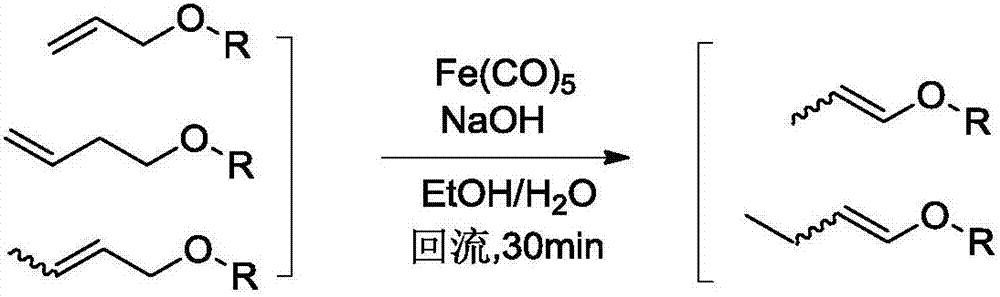
Catalyst systems for use in continuous flow reactors and methods of manufacture and use thereof
InactiveUS20160175829A1Preparation by isomerisationCarboxylic acid esters preparationContinuous flowMonomer
The present application provides a composite material and system for use in a heterogeneous flow reactor, which can include: a catalytic polymeric framework comprising catalyst-containing monomeric units each separated by at least one non-catalyst-containing monomeric unit; and a solid support material, wherein the catalytic polymeric framework is covalently or non-covalently immobilized on or in the support material. Each catalyst-containing monomeric subunit in the polymeric framework comprises a transition metal bound to a catalyst ligand. Also provided are methods of manufacture and use of the catalyst system and composite material.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
Method for the production of isopulegol
ActiveUS7550633B2Suppress formationOxygen-containing compound preparationPreparation by isomerisationVinyl etherKetone
The present invention relates to a process for the preparation of isopulegol of formula (I):comprising the cyclization of citronellal of formula (II):in the presence of a tris(aryloxy)aluminum catalyst, wherein the cyclization is carried out in the presence ofI. at least one acid and / orII. at least one compound selected from the group comprising carboxylic anhydrides, aldehydes, ketones and vinyl ethers.
Owner:BASF SE
Preparation of immobilization molybdate catalyst
InactiveCN101214435ALong-term activityHave mechanical strengthPreparation by isomerisationOrganic-compounds/hydrides/coordination-complexes catalystsSilica gelHigh activity
The invention relates to a method of preparing a solid molyadate catalyst. Ammonium paramolybdate, i.e. (NH4)6MoO24.4H2O is taken as the active component of the catalyst, and then fixed on a compound carrier which is formed by that silica gel or sillitin forcibly absorbs chitosan. 0.1 percent to 0.5 percent (NH4)6MoO24.4H2O water solution is taken to react with particles of the compound carrier in 70 DEG C to 90 DEG C ethyl alcohol under 70 DEG C to 85 DEG C (V / V) temperature for 1 to 4 hours, which is dried to prepare the solid catalyst with high activity. The prepared solid molyadate catalyst is used for isomeric 50 percent (W / W) glucose solution, which is reusable for at least 10 times, with the activity still above 80percent.
Owner:广西南宁化学制药有限责任公司
Catalytic hydrogenation synthesis method for prenyl alcohol
InactiveCN105175229AHigh activityAvoid inactivationPhysical/chemical process catalystsPreparation by isomerisationDistillationSynthesis methods
The invention provides a catalytic hydrogenation synthesis method for prenyl alcohol. The method comprises the following steps: under the action of a solid base catalyst, adding paraformaldehyde with a concentration of 93 to 97% and tert-butyl alcohol used as a solvent into a reaction vessel, injecting nitrogen into the reaction vessel to replace air in the reaction vessel, then adding isobutene into the reaction vessel, carrying out stirring, then carrying out a continuous reaction at 180 to 220 DEG C for 3 to 5 h and carrying out cooling, discharging and distillation; collecting prenyl alcohol obtained after distillation; and adding 3-methyl-buten-l-ol into a fixed-bed reactor and carrying out catalytic hydrogenation at 50 to 100 DEG C under the catalysis of a silica gel-loaded Pd catalyst promoted by Se and Ce so as to prepare prenyl alcohol. Thus, the method provided by the invention can improve reaction yield and selectivity of prenyl alcohol, overcomes the problems of corrosion and pollution existing in acidic catalysis, shortens reaction time and reduces production cost.
Owner:SHANDONG CHENGTAI CHEM IND
Synthetic method of vanadium-silver-molybdenum phosphate catalyst and method to catalytically prepare prenol and 3-methyl-2-butenal
ActiveCN108404944APreparation by isomerisationPhysical/chemical process catalystsIsobutanolContinuous use
The invention discloses a synthetic method of vanadium-silver-molybdenum phosphate catalyst and a method to catalytically prepare prenol and 3-methyl-2-butenal. The catalyst is prepared by: (1) addingvanadium, silver, molybdenum, phosphorus, benzyl alcohol and isobutanol according to certain molar ratios into a reactor, stirring well, allowing reaction at 100-130 DEG C for 8-10 hours, filtering,and drying filter cake at 150-200 DEG C for 4-5 hours; tableting the solid with a press machine, and activating for 10 hours under the conditions of vapor volume ratio of 70-90%, air volume ratio of 10-30% and temperature of 400-450 DEG C. prenol and 3-methyl-2-butenal are continuously produced at the temperature of 350-450 DEG C from 3-methyl-3-butenyl-ol under the vanadium-silver-molybdenum phosphate catalyst. The vanadium-silver-molybdenum phosphate catalyst is suitable for continuous use for longer than half a year and has unchanged catalytic activity.
Owner:CHINA CATALYST HLDG CO LTD
Preparation method of lithium phosphate catalysts
A new method for preparing lithium phosphate catalysts is disclosed. The method comprises precipitating a lithium phosphate from a mixture comprising a first aqueous solution which contains lithium and sodium ions and a second aqueous solution which contains phosphate and borate ions. The resultant lithium phosphate catalyst has increased activity and selectivity in the isomerization of an alkylene oxide to the corresponding allylic alcohol.
Owner:LYONDELL CHEM TECH LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com