Process for synthesizing sex pheromone of pine caterpillar
A synthetic method and technology of pine caterpillars, applied in the field of pine caterpillar sex pheromone synthesis, can solve the problems of harsh reaction conditions, low yield, stereoselectivity and low yield, and achieve mild reaction conditions, low cost, high The effect of industrial production value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
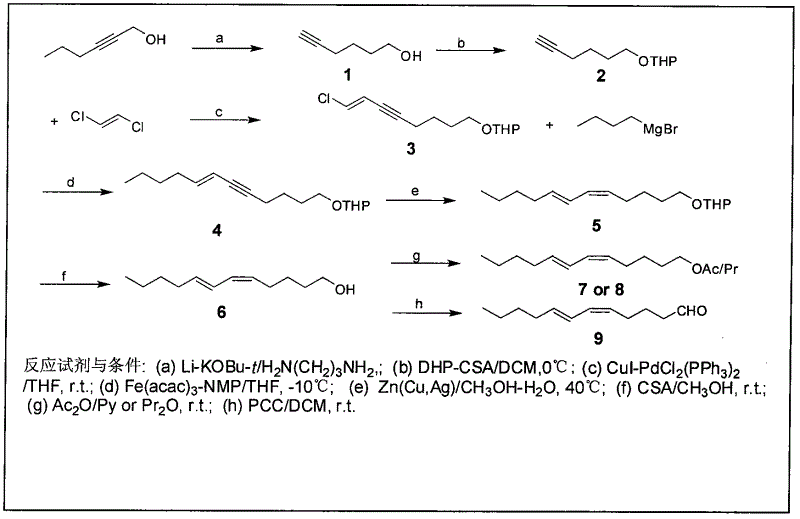
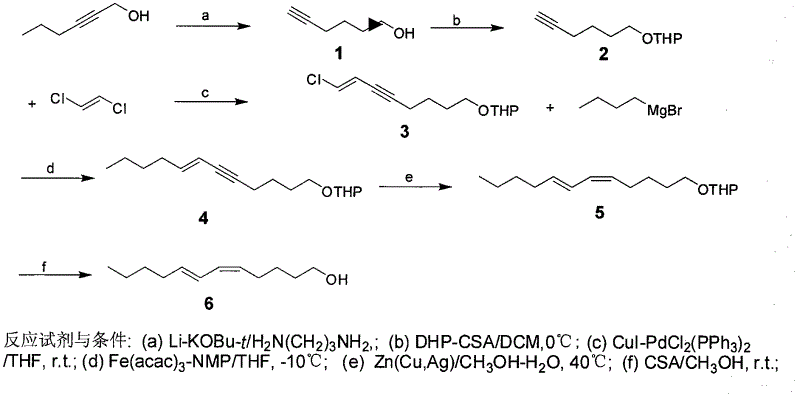
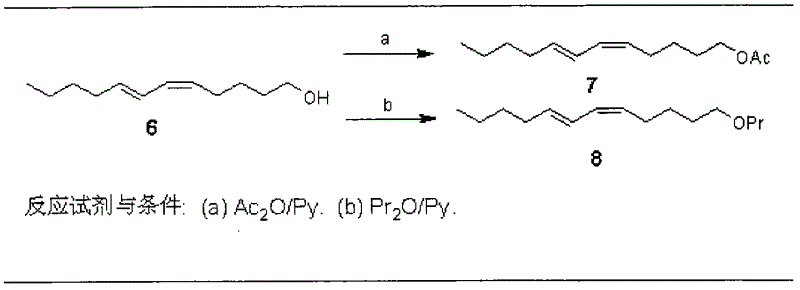
[0026] The present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments.
[0027] Pine caterpillar sex pheromone compounds contain four compounds: (5Z, 7E)-dodecadienol, (5Z, 7E)-dodecadiene acetate, (5Z, 7E)-dodecadiene Propionate and (5Z, 7E)-dodecadienal, in which the concentration ratio of Dendrolimus punctatus is (5Z, 7E)-dodecadienal acetate: (5Z, 7E)- Dodecadienoate: (5Z, 7E)-dodecadienol is 25:10:28, and the concentration ratio of the Chinese pine caterpillar (D. tabulaeformis) is (5Z, 7E)-dodecadienol Ethyl acetate: (5Z, 7E)-dodecadienyl acrylate: (5Z, 7E)-dodecadienol is 100:29:47, and the concentration ratio of the red pine caterpillar (D.spectabilis) is (5Z, 7E)-dodecadiene acetate: (5Z, 7E)-dodecadienyl acrylate: (5Z, 7E)-dodecadienol is 3:25:100, Yunnan The concentration ratio of pine caterpillar (D.houi) is (5Z, 7E)-dodecadiene acetate: (5Z, 7E)-dodecadienal: (5Z, 7E)-dodecadiene Alcohol is 5.8:0.8:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com