Piperitol isomerization catalyst and method for continuously obtaining L, D-piperitol
A technology of menthol and catalyst, which is applied in the field of compound preparation, can solve the problems such as complex catalyst preparation procedures, and achieve the effects of easy preparation, high yield and simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: prepare Raney nickel catalyst
[0041] Raney nickel catalyst among the present invention is made up of the nickel of 90% by weight and 10% aluminum (percentage by weight), and it can be prepared by QB / TH08-1997 standard, also can directly buy on the market (as from Shijiazhuang Hengrun Technology Limited purchase).
Embodiment 2
[0042] Embodiment 2: Preparation of menthol isomer mixture by hydrogenation of thymol
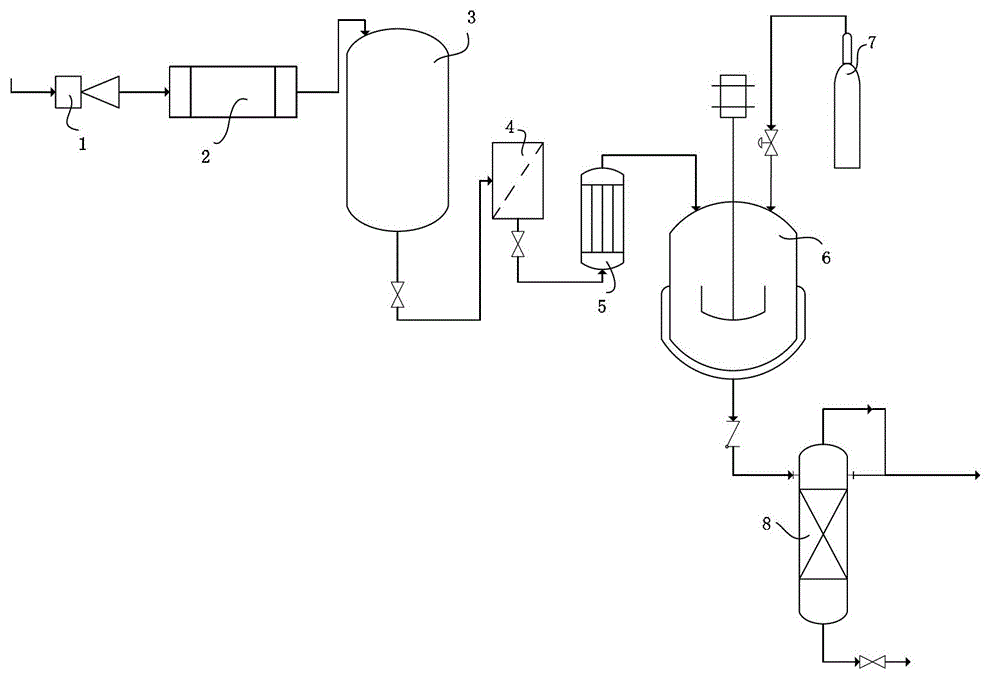
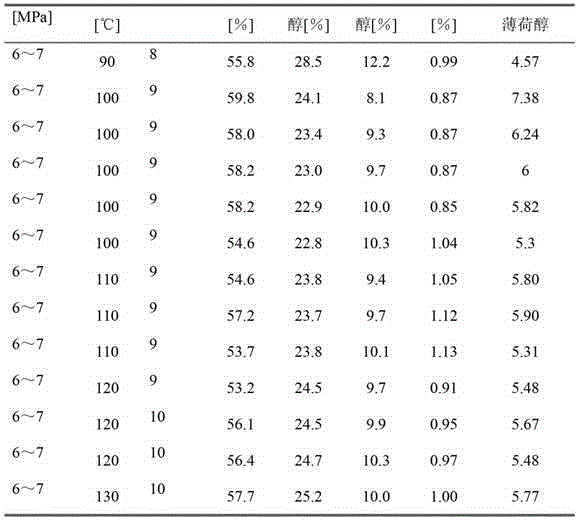
[0043] In a 5L reaction kettle, add 3L cyclohexane into the autoclave, add 1Kg of thymol and 120g of Raney nickel catalyst (wet basis, prepared in Example 1) respectively, adjust the pH value to 8-10, seal, and pre- After replacing the air in the kettle with hydrogen, adjust the temperature to 90-130°C and the hydrogen pressure to 6-7 MPa, and vigorously stir the reaction for 40 minutes. The results are shown in Table 1. All contents are determined by GC-MASS.
[0044] Table 1 Thymol Hydrogenation Results
[0045]
[0046]
[0047] Menthol in Table 1 is the enantiomer of L-menthol and D-menthol, neomenthol is the enantiomer of L-neomenthol and D-neomenthol, and isomenthol is L-isomenthol and D- Enantiomers of isomenthol.
[0048] [%] is % by weight.
[0049] The hydrogenated Raney nickel catalyst needs to be recovered and reused 3 to 5 times.
[0050] L, D-menthol is separated by...
Embodiment 3
[0051] Embodiment 3: Catalyst preparation of menthol enantiomerization
[0052] Accurately weigh 29g of Ni(NO 3 ) 2 ·6H 2 O and 7.3g of Cu(NO 3 ) 2 ·3H 2 O poured into 210g γ-Al 2 o 3 Dissolve in 50mL of distilled water, then adjust the pH to 9 with concentrated ammonia water, then slowly add distilled water to 3L and let it stand for 9h to fully hydrolyze the metal ammonia salt solution; filter, dry, and wash with distilled water for 5~ 7 times until NO is completely removed 3 - Finally, dry it again and vacuum dry it for 18 hours; the catalyst should be reduced before use by placing the catalyst in an autoclave, and gradually increasing the hydrogen to 2 MPa and keeping it for 2 hours as the temperature gradually rises to 450°C. In this way, Ni-Cu / γ-Al is obtained 2 o 3 Catalyst (binary metal catalyst), the particle size of the catalyst is 5-8nm.
[0053] The above preparation method is based on the attachment insertion hydrolysis method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com