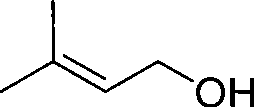
Method for continuously preparing 3-methyl-2-butenol
A technology of butenol and methyl, which is applied in the field of preparation of 3-methyl-2-butenol, an intermediate of spices, pharmaceuticals and pesticides, can solve the problems of high cost and low yield, and achieve the elimination of cost and raw materials Easy to obtain, less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0030] Example 1-3: Preparation of 3-methyl-2-butenol
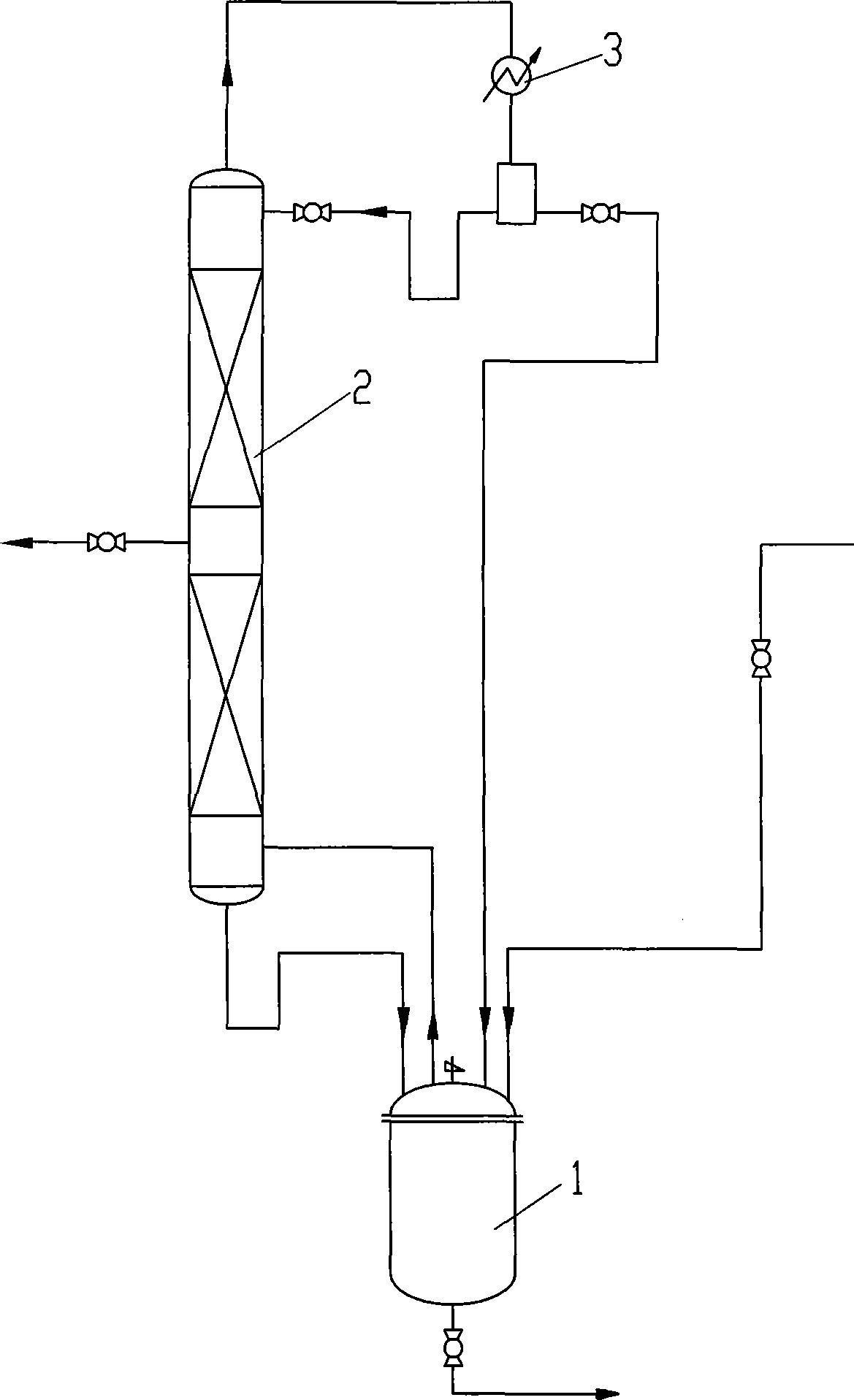
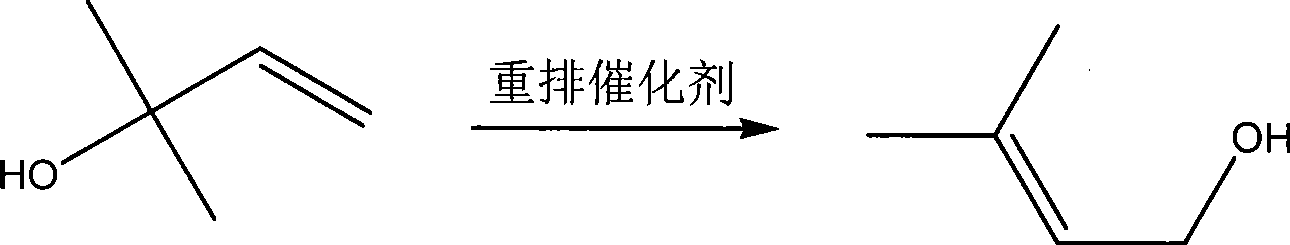
[0031] A reaction device of 250m1 reactor with mechanical stirring, 1.5m rectification tower and thermometer is used. The rectification tower has 30 theoretical plates, and there is a side-line outlet at 10 plates from the bottom of the tower. The feed port of the reaction kettle uses a metering pump to accurately feed 2-methyl-3-buten-2-ol. Put in 100g 2-methyl-3-buten-2-ol, vanadium oxytetraisopentenol ester OV (OC 5 H 9 ) 4 10.0g, the 2-methyl-3-buten-2-ol feed was started after the temperature was raised to 140°C, and the crude 3-methyl-2-butenol was discharged from the side discharge port, keeping the reaction temperature at 140- 150°C. The feeding is finished, and the crude 3-methyl-2-butenol is directly distilled out under reduced pressure after the reaction is completed, and combined with the output of the side thread. Table 1 shows the reaction results of Examples 1-3:
[0032] Table 1
[0033] Response para...
Embodiment 4-6
[0034] Example 4-6: Preparation of 3-methyl-2-butenol
[0035]A reaction device with a 250ml reactor with mechanical stirring, 2.0m rectification tower and thermometer is used. The rectification tower has 40 theoretical plates, and there is a side-line outlet at 10 plates from the bottom of the tower. The feed port of the reaction kettle uses a metering pump to accurately feed 2-methyl-3-buten-2-ol. Put in 100g 2-methyl-3-buten-2-ol, vanadium oxytetraisopentenol ester OV(OC 5 H 9 ) 4 10.0g, the 2-methyl-3-buten-2-ol feed was started after the temperature was raised to 140°C, and the crude 3-methyl-2-butenol was discharged from the side discharge port, keeping the reaction temperature at 140- 150°C. After half of the 2-methyl-3-butene-2-ol is fed, 10.0g of vanadium oxotetraisopentenol ester is added to complete the feeding. After the reaction is completed, 3-methyl is directly distilled out under reduced pressure. The crude -2-butenol is combined with the discharge of the side line...
Embodiment 6
[0038] After rectifying and separating the crude 3-methyl-2-butenol obtained in Example 6, 1254.1 g of 3-methyl-2-butenol was obtained, with a content of 98.2%; 2-methyl-3-butene was recovered. The 2-alcohol 951.6g, the content is 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com