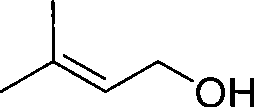
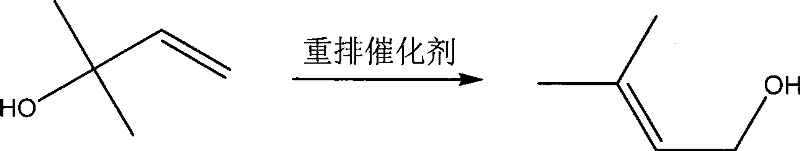
Method for continuously preparing 3-methyl-2-butenol
A kind of butenol, methyl technology, applied in the preparation of medicine and pesticide intermediate 3-methyl-2-butenol, perfume field, can solve the problems of high cost, low yield, etc., to eliminate cost, raw material Easy to obtain and reduce energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0030] Embodiment 1-3: Preparation of 3-methyl-2-butenol
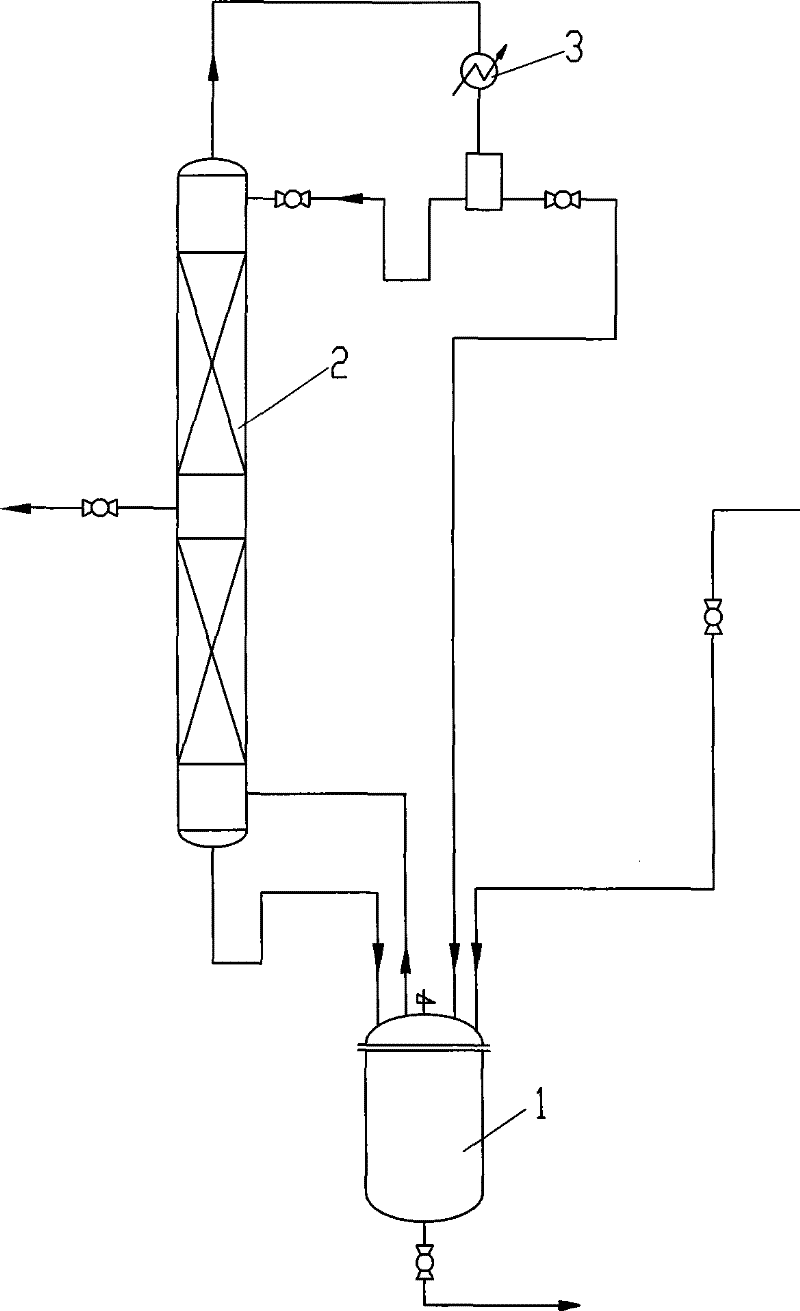
[0031] Use a kind of reaction device with the 250ml reactor of mechanical stirring, 1.5m rectifying tower, thermometer, the number of theoretical trays of rectifying tower is 30, there is a side line outlet at 10 trays from the bottom of the tower, in The feed port of the reactor realizes accurate feeding of 2-methyl-3-buten-2-ol through a metering pump. Drop into 100g2-methyl-3-butene-2-alcohol earlier, vanadium oxytetraprenol ester OV(OC 5 h 9 ) 4 10.0g, start 2-methyl-3-buten-2-alcohol feeding after being warmed up to 140 ℃, 3-methyl-2-butenol crude product discharges from the side line outlet, keep reaction temperature as 140- 150°C. After finishing the feed, the crude product of 3-methyl-2-butenol was distilled off directly under reduced pressure after the reaction was completed, and combined with the output from the side line. Table 1 is the reaction result of embodiment 1-3:
[0032] Table 1
[0033] ...
Embodiment 4-6
[0034] Embodiment 4-6: Preparation of 3-methyl-2-butenol
[0035] Use a kind of reaction device with the 250ml reactor of mechanical stirring, 2.0m rectifying tower, thermometer, the theoretical tray number of rectifying tower is 40, there is side line outlet at 10 trays from the bottom of the tower, in The feed port of the reactor realizes accurate feeding of 2-methyl-3-buten-2-ol through a metering pump. Drop into 100g2-methyl-3-butene-2-alcohol earlier, vanadium oxytetraprenol ester OV(OC 5 h 9 ) 4 10.0g, start 2-methyl-3-buten-2-alcohol feeding after being warmed up to 140 ℃, 3-methyl-2-butenol crude product discharges from the side line outlet, keep reaction temperature as 140- 150°C. After half of the 2-methyl-3-buten-2-ol is fed, 10.0 g of vanadium oxytetraprenol ester is added to finish the feeding. After the reaction is completed, the 3-methyl - Crude 2-butenol, combined with the side draw. Table 2 is the reaction result of embodiment 4-6:
[0036] Table 2
[0...
Embodiment 6
[0038] After the crude 3-methyl-2-butenol obtained in Example 6 was separated by rectification, 1254.1 g of 3-methyl-2-butenol was obtained, with a content of 98.2%; 2-methyl-3-butene- 2-alcohol 951.6g, content 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com