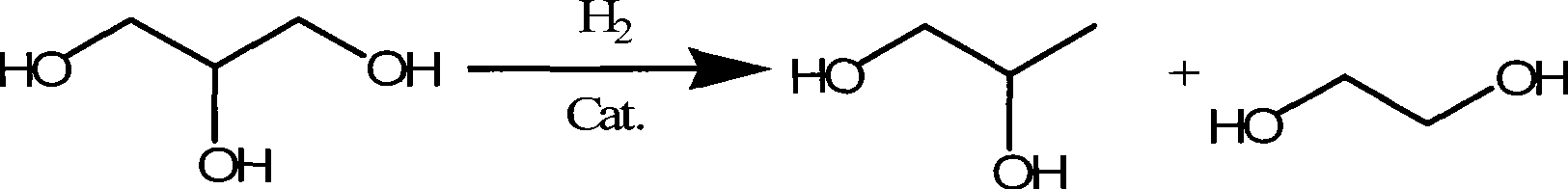
Method for hydrocracking glycyl alcohol
A technology for hydrocracking and glycerol, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, organic chemistry, etc. problem, to achieve the effect of high catalytic activity, low price and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of catalyst
[0028] For preparing catalyst of the present invention, carrier adopts coconut shell activated carbon, and its characteristics are as follows:
[0029] Specific surface: 1306.80m 2 / g; average pore size: 2.4nm; particle size: 80-100 mesh and dry before use.
[0030] Dissolve the soluble metal salt solution in water to prepare the impregnation solution with the required concentration, impregnate it into the carrier, keep it for 24 hours, and then dry it at a constant temperature of 120°C to make single-component or double-component with different loads and components Sub-supported metal catalysts (Table 1 is the prepared catalysts of different metal components). Then, by KBH 4 Reduction prepared into an amorphous catalyst.
[0031] Specifically as the preparation of No. 1 catalyst: take by weighing the solution of nickel nitrate and cerium nitrate (Ni: Ce=8: 1, mass ratio), add the activated carbon after drying (the loading...
Embodiment 2-10
[0034] The activity evaluation of embodiment 2-10 catalyst
[0035] The following examples adopt the following general method to prepare low-carbon diols by hydrocracking glycerol with the catalyst of Example 1.
[0036] The catalytic hydrocracking performance evaluation of glycerol was carried out in a 600ml autoclave, the raw material was 150g of 25% mass concentration glycerol aqueous solution, and the amount of catalyst before reduction was 7g. The hydrogen pressure is 5MPa, the reaction temperature is 200°C, and the reaction time is 6h.
[0037] The reaction products were analyzed by gas chromatography.
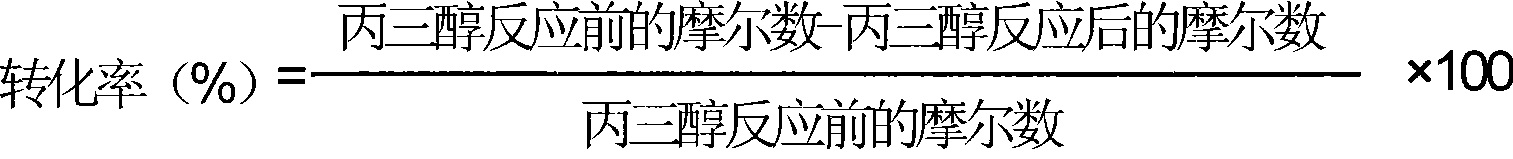
[0038] The transformation rate of glycerol adopts following formula to calculate:
[0039]
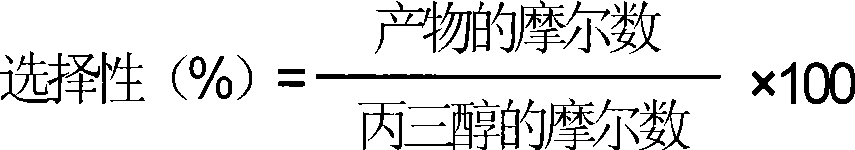
[0040] The selectivity of the product is calculated using the following formula:
[0041]
[0042] Table 3 Effect of catalysts with different compositions on the hydrocracking performance of glycerol
[0043]
[0044] Influence of different proportions of components ...
Embodiment 18-20
[0052] Embodiment 18-20 Effect of reaction time on glycerol hydrocracking performance
[0053] The influence of different time periods on the hydrocracking performance of glycerol was studied. The catalyst number was 8. Other reaction processes were the same as in Examples 2-7. The test results were as follows.
[0054] Table 8 Effect of different reaction time on the hydrocracking performance of glycerol
[0055]
[0056] Embodiment 21-22 Effect of reaction temperature on glycerol hydrocracking performance
[0057] The influence of different temperatures on the hydrocracking performance of glycerol was studied. The reaction time was 8 hours, the catalyst number was 8, and other reaction processes were the same as in Examples 2-7. The test results are as follows.
[0058] Table 9 Effects of different reaction temperatures on glycerol cracking
[0059]
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com