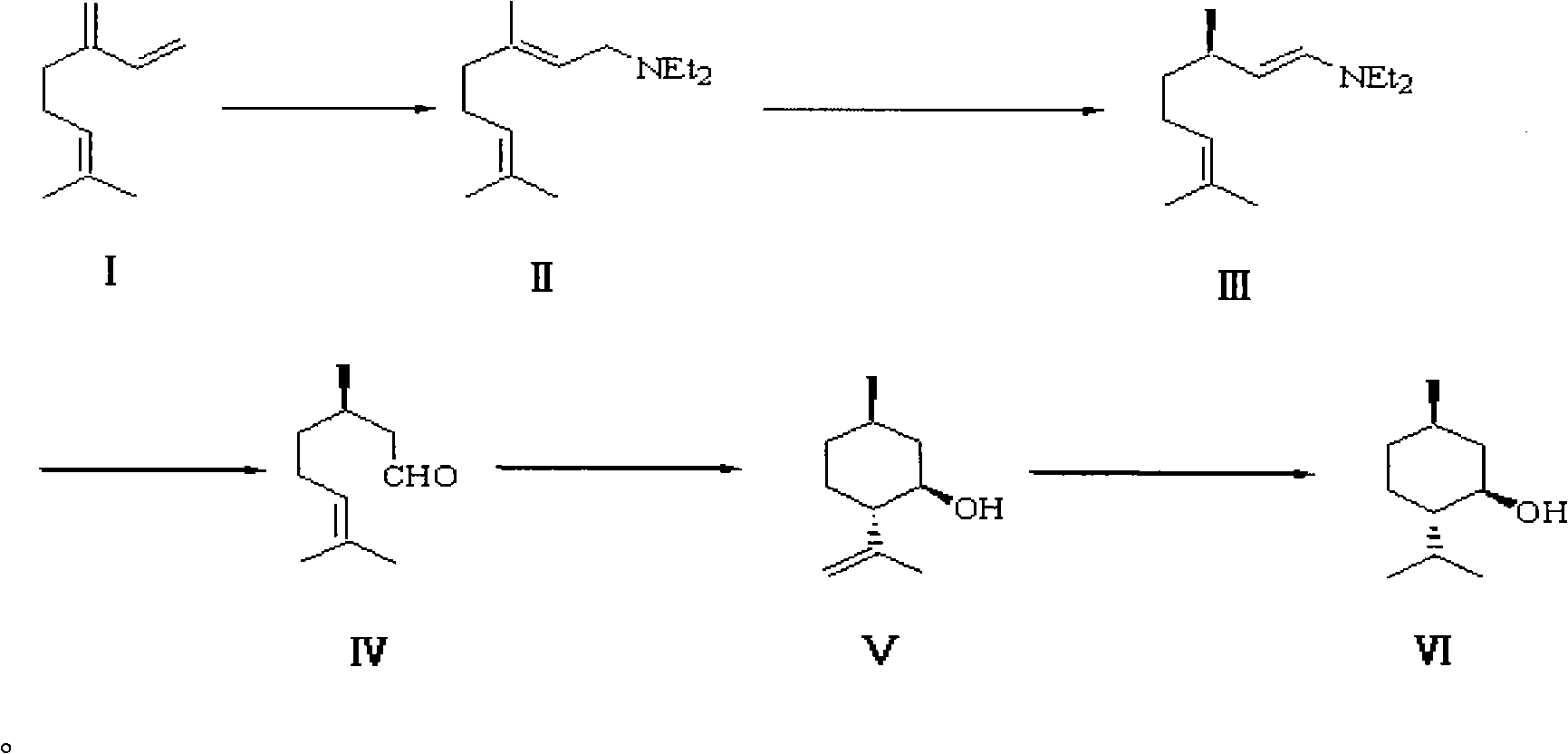
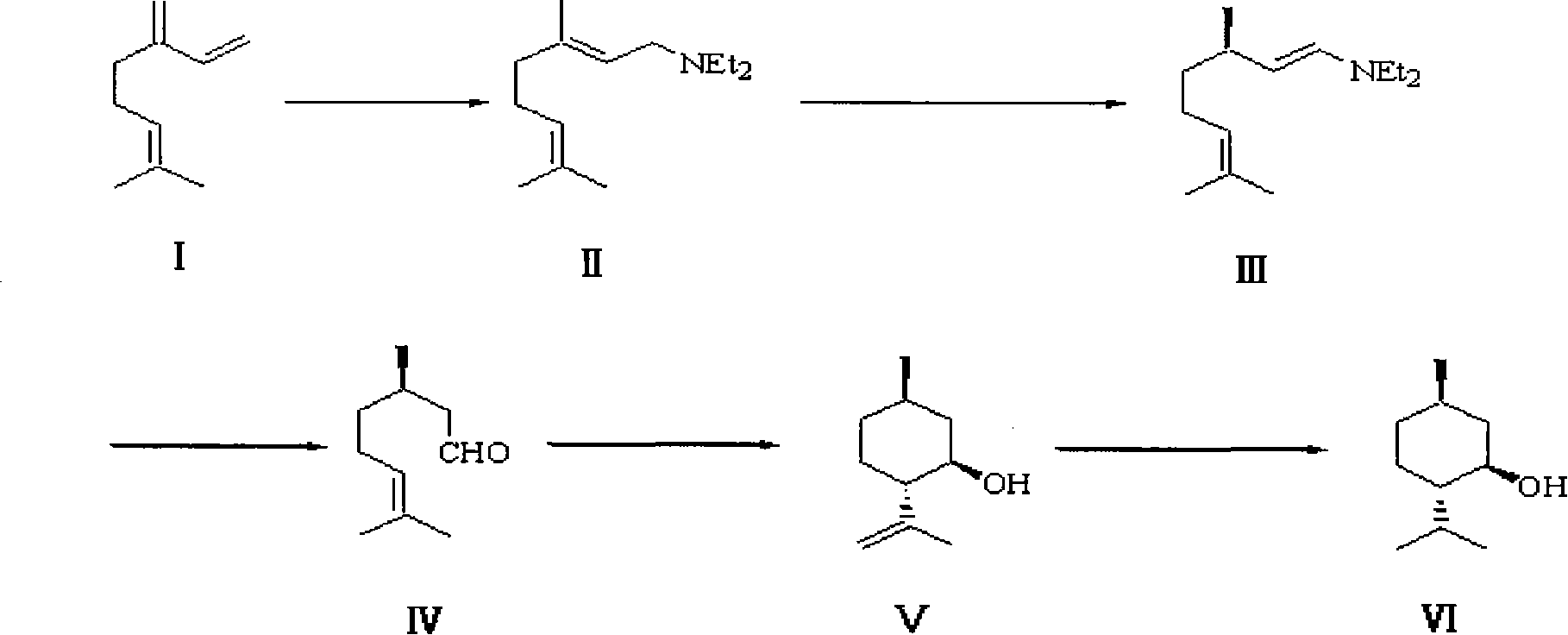
Method for synthesizing L-menthol
A synthesis method and menthol technology, applied in the synthesis field of L-menthol, can solve the problems of difficult industrial application, complicated process, low yield and the like, and achieve the effects of high yield, simple process and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthesis of embodiment 1 L-menthol
[0024] 1. Amination reaction.
[0025] Replace the 1L three-necked bottle equipped with electromagnetic stirring, reflux condenser, constant pressure dropping funnel, suction head and thermometer into a nitrogen atmosphere, and add freshly steamed myrcene (80%, 82-84 ° C / 4mmHg, 173.3g, 218mL, 1.02mol), diethylamine (140g, 200mL, 1.91mol), slowly add n-BuLi (93mL, 2.2M in ether, 0.205mol, 20mol%) dropwise under stirring, and add dropwise for 70min After completion, the water bath was heated to 60° C. and stirred for 7 hours, then the heating was stopped, and the system was allowed to return to room temperature naturally, and slowly poured into 1 L of ice water, stirring continuously during this period. The layers were separated, the aqueous phase was extracted with ether (3×300 mL), the organic phases were combined, washed with 500 mL of water, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filt...
Embodiment 2
[0042] The synthesis of embodiment 2 L-menthol
[0043] 1. Amination reaction.
[0044] Replace the 1L three-necked bottle equipped with electromagnetic stirring, reflux condenser, constant pressure dropping funnel, suction head and thermometer into a nitrogen atmosphere, and add freshly steamed myrcene (80%, 82-84 ° C / 4mmHg, 173.3g, 218mL, 1.02mol), diethylamine (140g, 200mL, 1.91mol), slowly add n-BuLi (46mL, 2.2M in ether, 0.102mol, 10mol%) dropwise under stirring, and add dropwise for 50min After completion, the water bath was heated to 50° C. and stirred for 6 h, then the heating was stopped, and the system was allowed to return to room temperature naturally, and slowly poured into 1 L of ice water, while stirring continuously. The layers were separated, the aqueous phase was extracted with ether (3×300 mL), the organic phases were combined, washed with 500 mL of water, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, and dieth...
Embodiment 3
[0061] The synthesis of embodiment 3 L-menthol
[0062] 1. Amination reaction.
[0063] Replace the 1L three-necked bottle equipped with electromagnetic stirring, reflux condenser, constant pressure dropping funnel, suction head and thermometer into a nitrogen atmosphere, and add freshly steamed myrcene (80%, 82-84 ° C / 4mmHg, 173.3g, 218mL, 1.02mol), diethylamine (140g, 200mL, 1.91mol), slowly add n-BuLi (93mL, 2.2M in ether, 0.205mol, 20mol%) dropwise under stirring, add dropwise for 50min After completion, the water bath was heated to 50° C. and stirred for 5.5 hours, then the heating was stopped, and the system was allowed to return to room temperature naturally, and slowly poured into 1 L of ice water, stirring continuously during this period. The layers were separated, the aqueous phase was extracted with ether (3×300 mL), the organic phases were combined, washed with 500 mL of water, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com