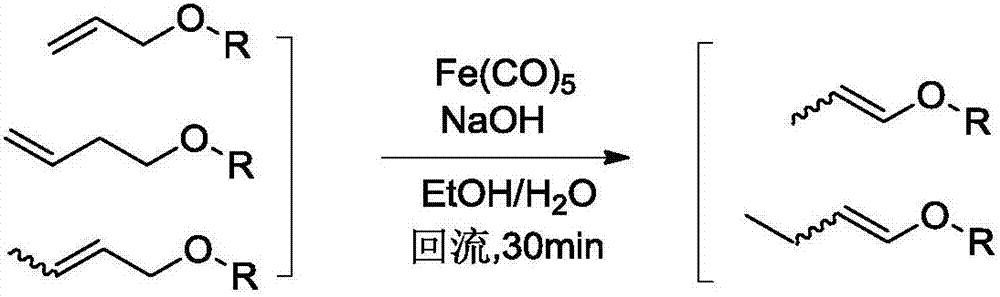
Method for preparing 3-methyl-2-butenol
A technology of butenol and methyl, which is applied in the field of preparing 3-methyl-2-butenol to achieve the effects of improving selectivity, increasing energy consumption, and avoiding by-products of hydrogenation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
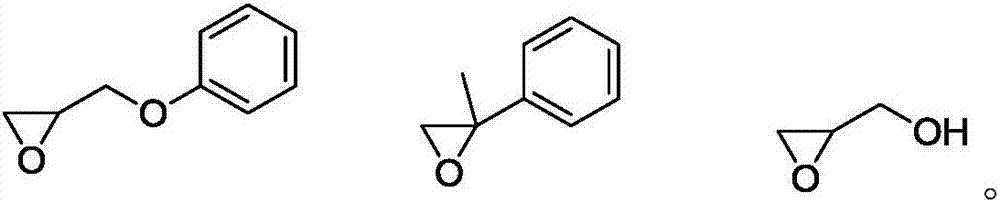
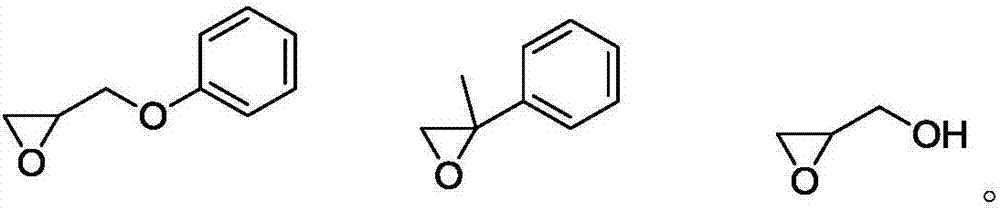
[0045] Under the protection of nitrogen atmosphere, 5 g (10 mmol) of triiron dodecacarbonyl and 0.75 g (5 mmol) of propylene oxide phenyl ether were mixed and stirred at room temperature for 24 h, then 5 g (50 mmol) of triethylamine was added and stirred at room temperature for 12 h. The mixture was transferred to an autoclave and 258 g (3 mol) of 3-methyl-3-butenol and 50 g of 18-crown-6 were added. The reactor was replaced 10 times with nitrogen containing 5000 ppm of carbon monoxide, the temperature of the reactor was raised to 80° C., the pressure was 0.5 MPa, and the reactor was stirred for 2 hours. The organic phase was analyzed by gas chromatography. The conversion was 47.3%, and the selectivity was 98.8%.
Embodiment 2
[0047] Under the protection of nitrogen atmosphere, 2g (10mmol) of iron pentacarbonyl and 0.65g (5mmol) of 2-phenylpropylene oxide were mixed and stirred at room temperature for 12h, then 8g (100mmol) of pyridine was added and stirred at room temperature for 6h. The mixed solution was transferred to the autoclave and added 17.2g (0.2mol) of 3-methyl-3-butenol and
[0048] 150 g macrogol 200. The reactor was replaced with nitrogen containing 10 000 ppm of carbon monoxide for 10 times, the temperature of the reactor was raised to 70° C., the pressure was 0.5 MPa, and the reactor was stirred for 24 hours. The organic phase was analyzed by gas chromatography. The conversion was 79.8%, and the selectivity was 99.0%.
Embodiment 3
[0050] Under the protection of nitrogen atmosphere, 3.6g (10mmol) of nonacarbonyl ferric iron and 0.15g (2mmol) of 3-hydroxy-1,2-propylene oxide were mixed and stirred at room temperature for 1h, and then 2g (20mmol) of triethylamine was added, Stir at room temperature for 1 h. The mixture was transferred to an autoclave and 172 g (2 mol) of 3-methyl-3-butenol and 200 g of polypropylene glycol 400 were added. The reactor was replaced 10 times with nitrogen containing 5 000 ppm of carbon monoxide, the temperature of the reactor was raised to 80° C., the pressure was 0.5 MPa, and the reactor was stirred for 0.5 h. The organic phase was analyzed by gas chromatography. The conversion was 51.3%, and the selectivity was 85.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com