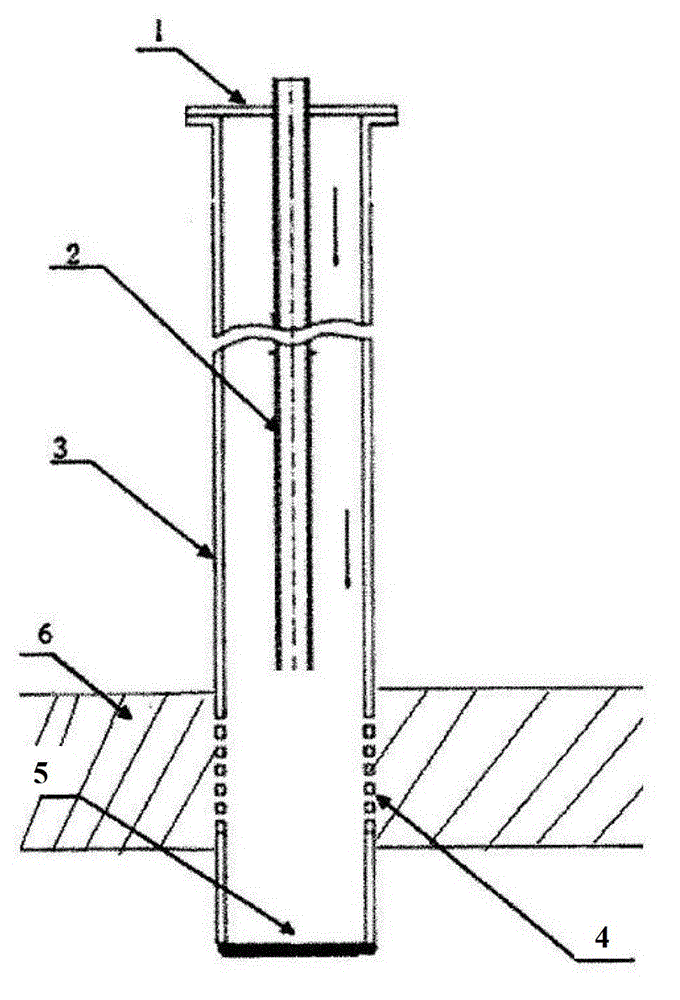
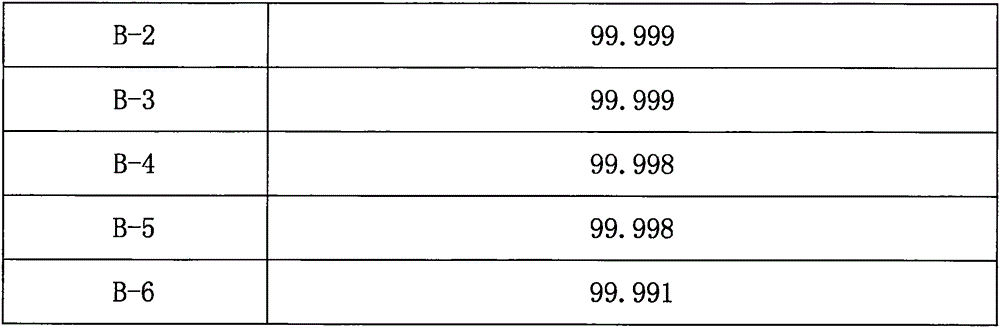
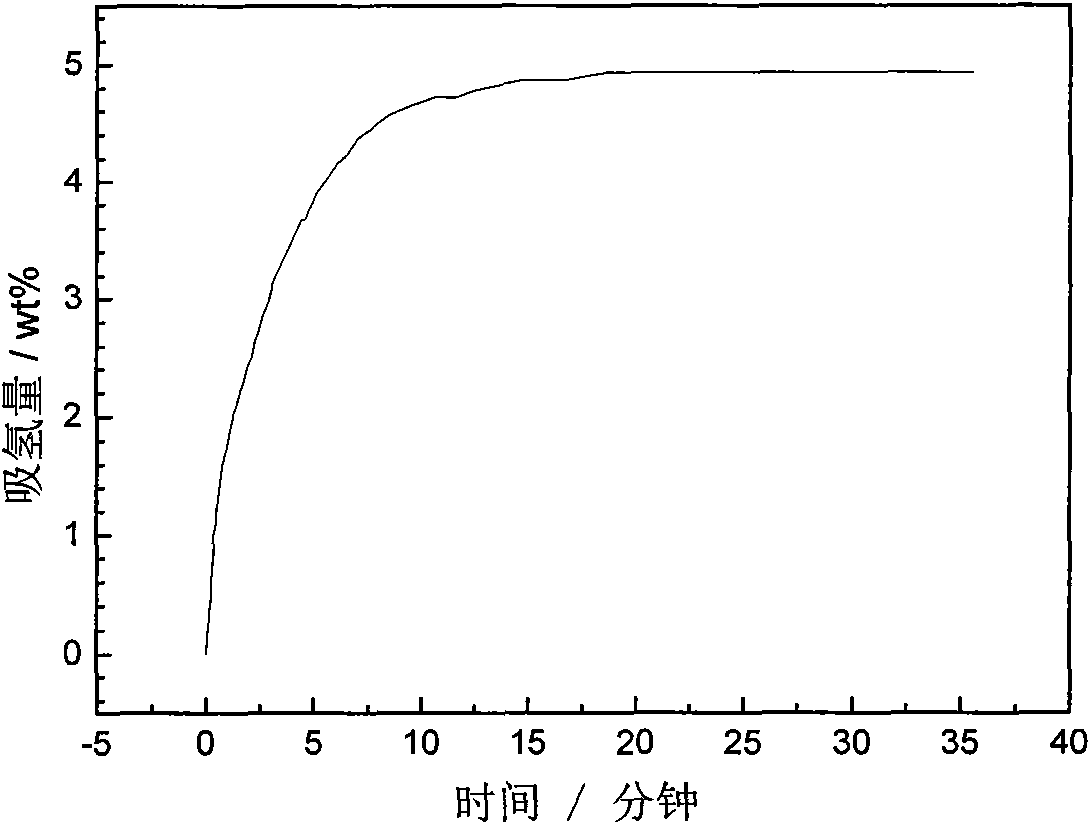
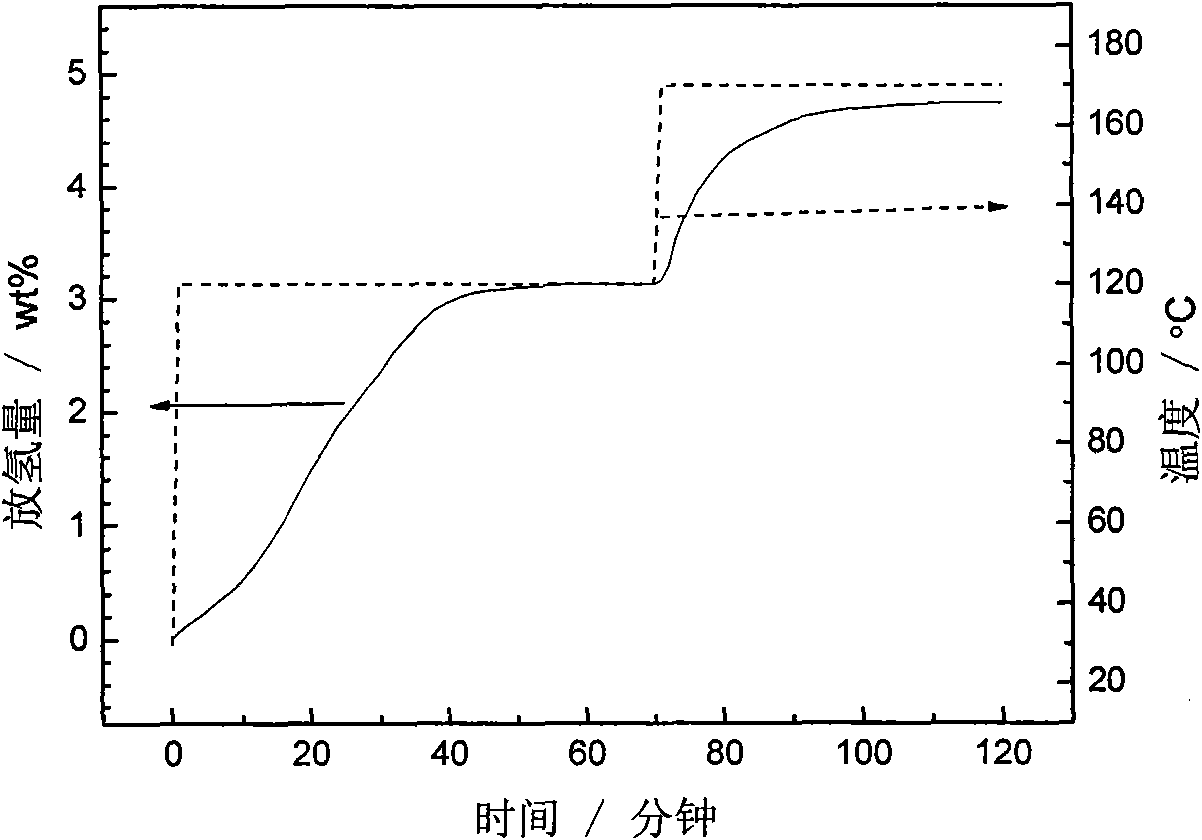
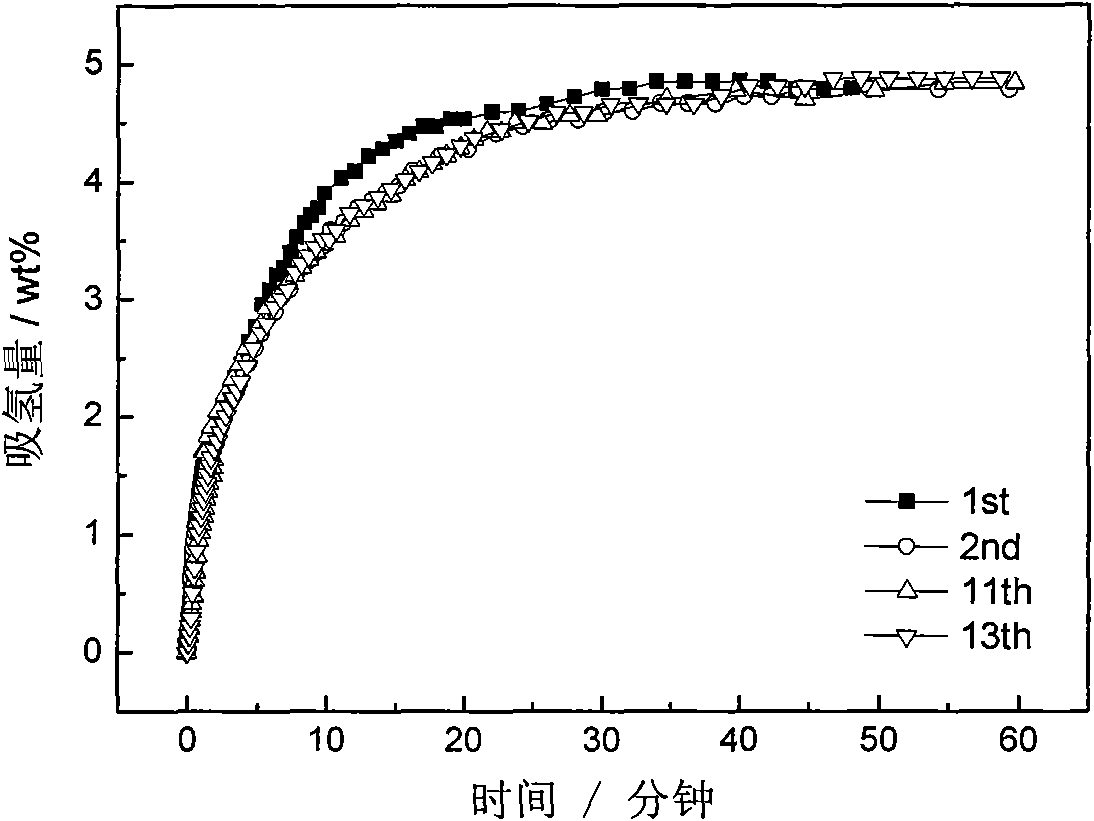
Patents
Literature
36 results about "Sodium aluminum hydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Process for production of sodium borohydride from sodium aluminum hydride with recycle of byproducts
A process for production of sodium borohydride. The process comprises the steps of: (a) combining a boric acid ester, B(OR)3 and sodium aluminum hydride to produce sodium borohydride and Al(OR)3; and (b) combining Al(OR)3 and sulfuric acid to produce alum and ROH.
Owner:ROHM & HAAS CO
Hydrogenation heat gas chemical yield increasing solution component applied to shallow well
ActiveCN102942913AHigh porosityImprove permeabilityFluid removalDrilling compositionPorosityAmmonium nitrate
The invention belongs to the technical field of oil exploitation, and relates to a hydrogenation heat gas chemical yield increasing solution component capable of increasing permeability of a shallow well and a near wellbore region. The solution component comprises first solution and second solution, the mass ratio of the first solution to the second solution is 1:1, the first solution comprises 37-43% of ammonium nitrate NH4NO3, 23-26% of urea CO(NH2)2, 5-8% of decaborane B10H14, 1-2% of glucose C6H12O6, 0.5-1.5% of methenamine (CH2)6N4 and 19.5-33.5% of water. The second solution comprises 44-48% of sodium nitrate NaNO3, 15-20% of sodium nitrite NaNO2, 17-21% of sodium aluminum hydride NaAlH4 and 15-19% of tetrachloroethylene C2Cl4. By the aid of the solution component, porosity of an ore bed can be increased, accordingly, permeability is increased, and the yield can be increased by 2-10 times.
Owner:吉林贯通能源科技有限责任公司
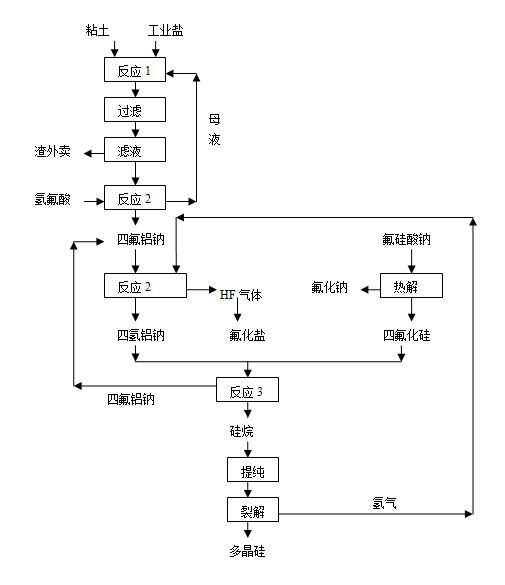
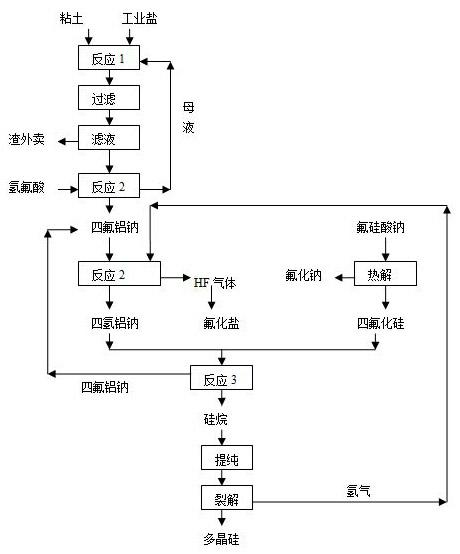
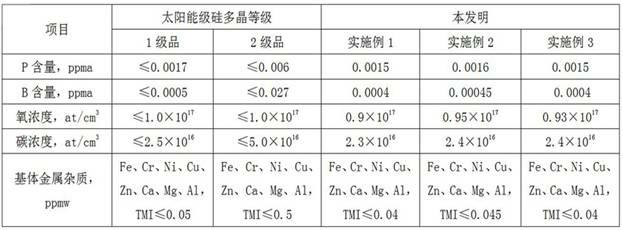
Method for producing polycrystalline silicon by utilizing sodium fluosilicate byproduct of phosphate fertilizer
ActiveCN102070144AReduce manufacturing costLow impurity contentSilicon hydridesSilicon tetrafluorideMaterials science
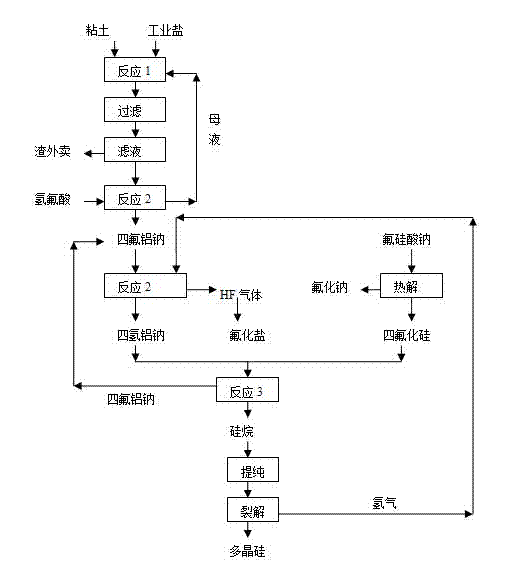
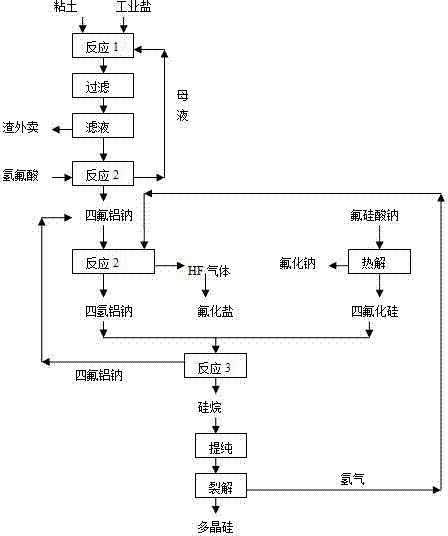
The invention relates to a method for producing polycrystalline silicon by utilizing a sodium fluosilicate byproduct of a phosphate fertilizer. The sodium fluosilicate byproduct of the phosphate fertilizer is subjected to paralysis to form silicon tetrafluoride and sodium aluminum hydride, which serve as raw materials to prepare silane, and the silane is cracked to form the polycrystalline silicon and hydrogen, wherein the hydrogen is circulated and used for hydrogenating sodium aluminum tetrafluoride. A hydrofluoric acid byproduct generated in the reaction process is used for producing fluoride salt products. In the method for producing the polycrystalline silicon, the byproduct is taken as a raw material, the price is low, the process is simple, easy to operate, environment-friendly and energy-saving; the produced polycrystalline silicon has low impurity content and high purity; in addition, the hydrofluoric acid byproduct in the production can be used for producing the fluoride salt products, so that the production cost is further reduced, and the method has better economic, social and environmental benefits.
Owner:DO FLUORIDE CHEM CO LTD
Method for purifying electron-grade hydrogen chloride
ActiveCN106044710AImprove the performance of hydrogen adsorptionImprove adsorption capacityChlorine/hydrogen-chloride purificationSorbentIon exchange
The invention relates to a method for purifying electron-grade hydrogen chloride. The method comprises the following steps: carrying out ion exchange between highly basic polystyrene macroporous ion exchange resin with sodium aluminum hydride NaAlH4 to generate an aluminum hydride resin adsorbent; loading 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, so that the performances of adsorbing hydrogen, carbon dioxide and other impurities are improved.
Owner:ZHEJIANG BRITECH CO LTD
Catalyst of sodium aluminum hydride coordination oxide and preparation method thereof
InactiveCN101642703ANo lossImprove the kinetic performance of hydrogen absorption and desorptionHydrogen productionMetal/metal-oxides/metal-hydroxide catalystsCeriumBall mill
The invention relates to a catalyst of sodium aluminum hydride coordination oxide and a preparation method thereof. A chemical general formula of the catalyst is RExAly, wherein x is smaller than 10 but larger than or equal to 1, and y is smaller than 20 but larger than or equal to 1. In the formula, the RE is Sc, Y, La, Ce, Pr, Sm, Nd, Ml (lanthanum-rich mixed tombarthite) or Mm (cerium-rich mixed tombarthite). The preparation method is as follows: according to a chemical amount proportion of the RExAly, RE and the Al block-shaped metal raw materials are weighed and proportionally mixed; theraw materials are then melted into an RExAly alloy cast ingot under an argon protection atmosphere and then crushed and put into a high-power ball mill for ball milling; and then the RExAly catalyst with a particle size of micro-nanometer level is obtained. The preparation process of the catalyst is simple, the operation is easy, and the cost is low. The catalyst is used for reversible catalysis hydrogen storage of sodium aluminum hydride, greatly improves the dynamic performance of the sodium aluminum hydride in terms of hydrogen absorption and release and does not undergo elementary reactionwith a matrix hydrogen storage material group to generate inert byproducts so that the system reversible hydrogen storage amount is not damaged.
Owner:ZHEJIANG UNIV
Nano-catalyst of sodium aluminum hydride complex hydride as well as preparation method and application thereof
InactiveCN101406843AHigh activityImprove stabilityPhysical/chemical process catalystsMetal hydridesNano catalystBall mill
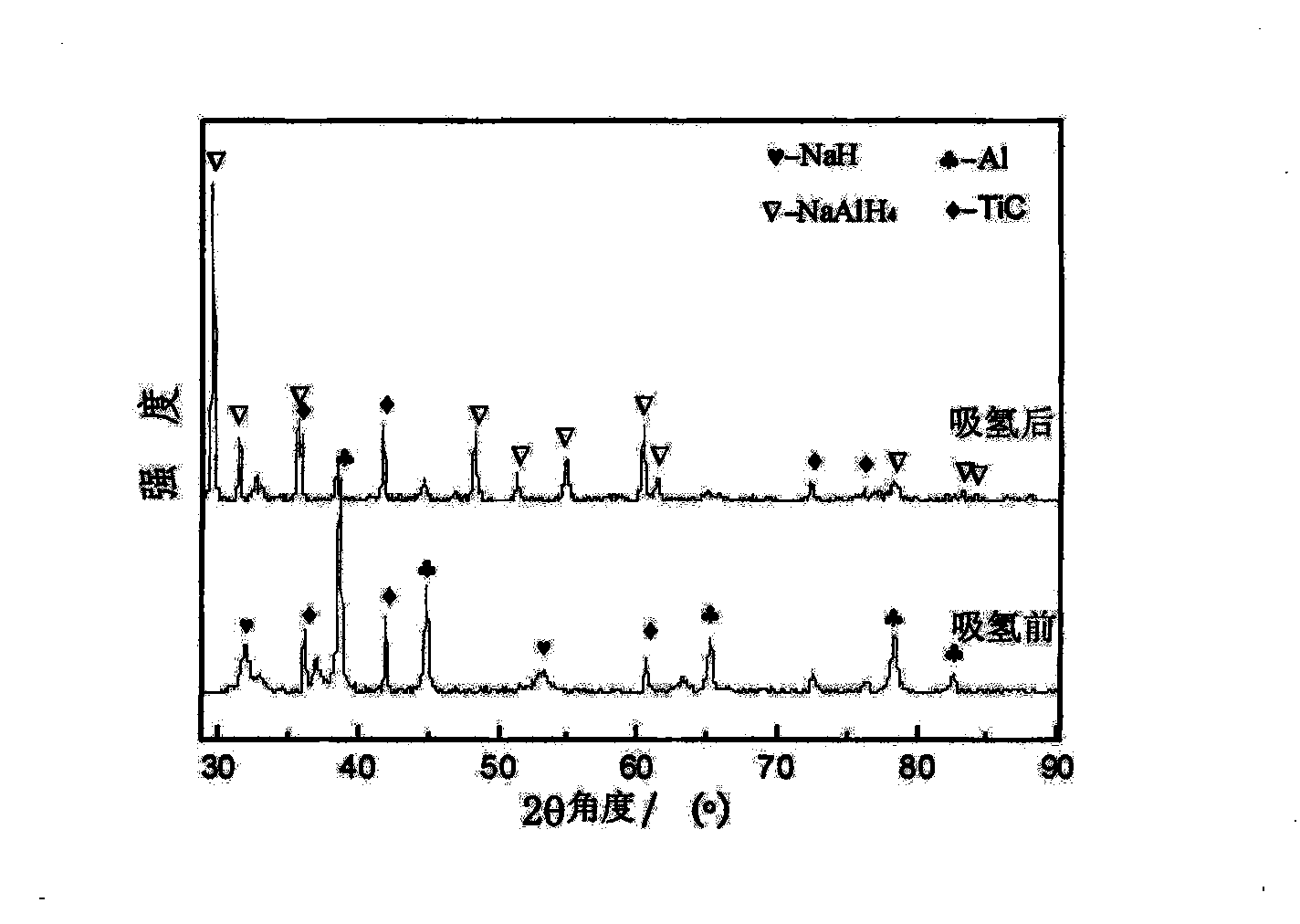
The invention relates to a nanometer catalyst for aluminum sodium hydride complex hydrides, as well as a preparation method and application thereof. The chemical formula of the catalyst is TixC1-x, wherein x is between 0.2 and 0.8. A method for preparing the catalyst comprises the following steps: according to the stoichiometric ratio of TixC1-x, simple-substance Ti and C powder are mixed, cold-pressed, molded, sintered, diffused and sintered in argon, cooled with a furnace, ground and ball-milled in a vibrating ball mill so as to obtain TixC1-x catalyst ultrafine powder. When the catalyst is used for reversibly-stored hydrogen of aluminum sodium hydride, the catalyst has good catalytic performance and can improve the capacity of the reversibly-stored hydrogen of the aluminum sodium hydride by more than 4.5 percent in weight. The catalyst has the advantages of simple preparation process, low cost and good activity and stability.
Owner:ZHEJIANG UNIV
Coordination hydride catalyzed reversible hydrogen storage materials and method of preparing the same
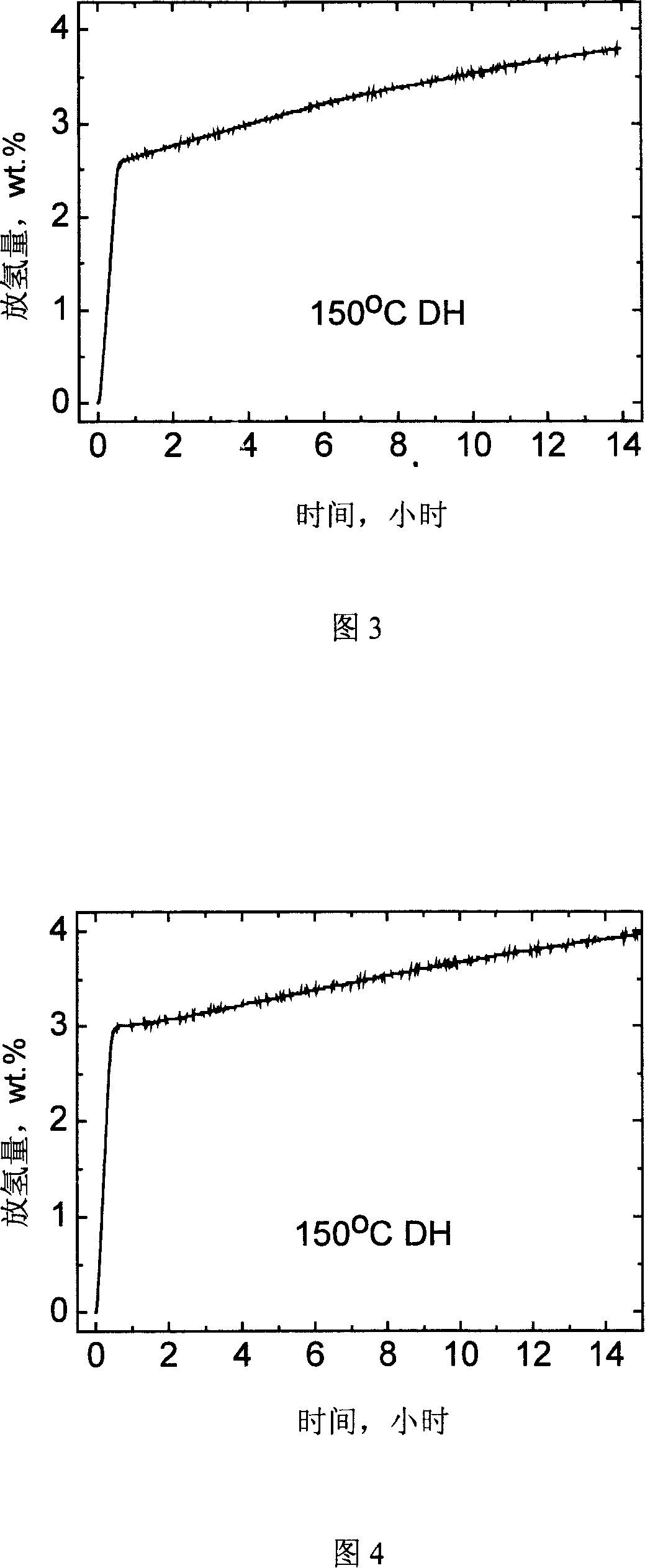
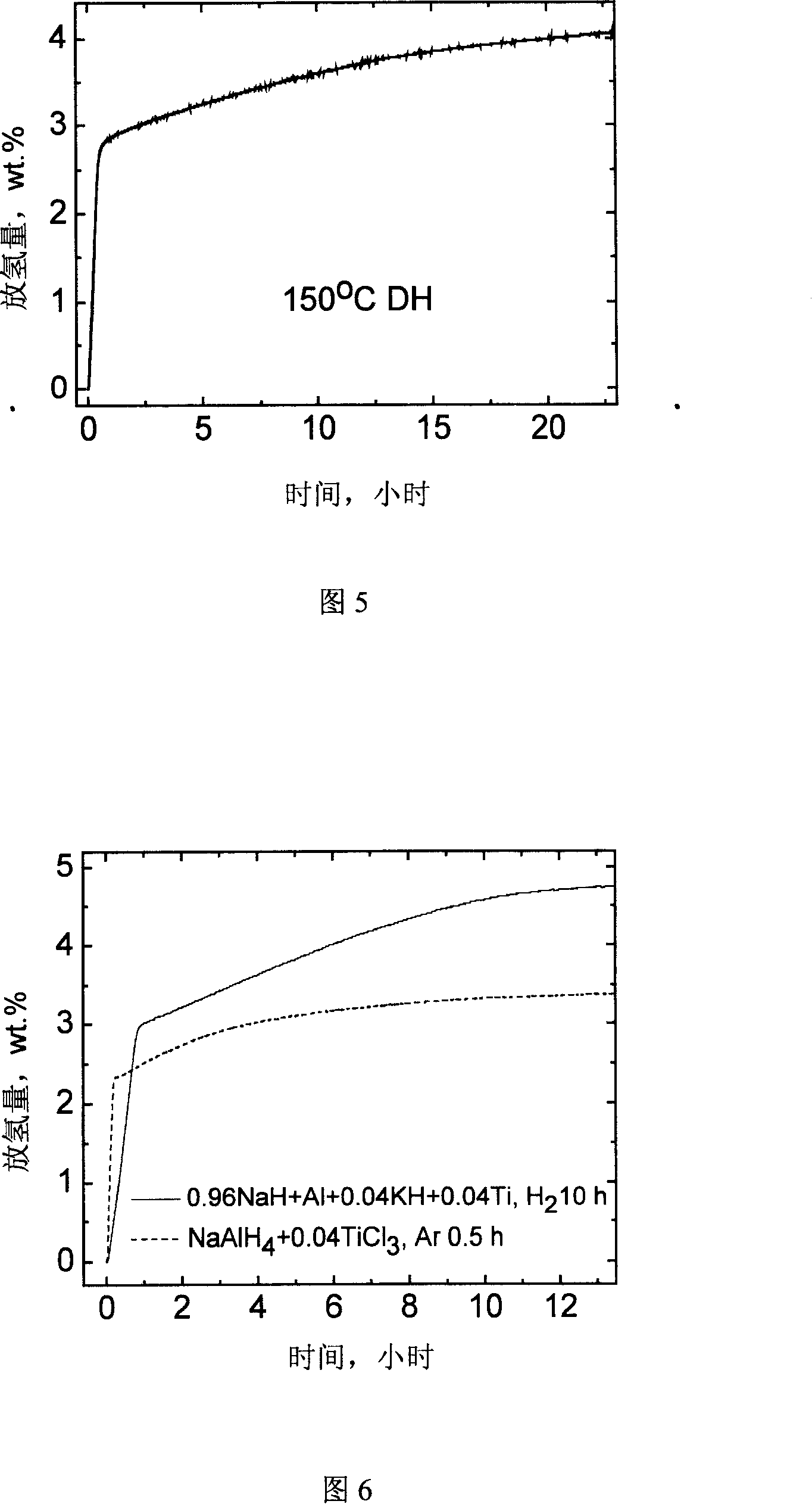
The invention relates to a novel high capacity hydrogen storage material, namely coordination hydride, the chemical formula is A(MH4)n. The invention adds titanium catalyst Ti(OC4H9)4 or TiC13 .1 / 3 AlCl3 in the classic coordination hydride lithium aluminium hydride and sodium aluminum hydride by the high energy ball mill method under the protection of hydrogen pressure, therefore reaching reversible storage hydrogen. The preparation method of the coordination hydride catalyzing reversible storage hydrogen seals the coordination hydride (LiA1H4 or NaA1H4), titanium catalyst Ti(OC4H9)4 or TiC13 .1 / 3 AlCl3 and steel ball in a stainless ball mill Pot, and mill ball with high energy under the protection of hydrogen pressure. The material can be used as a high capacity hydrogen storage material, the reversible hydrogen adsorption volume can reach above 4.0wt per cent at 150 DEG C., the reversible hydrogen adsorption volume to 0.1MPa hydrogen pressure also can reach above 3.0 wt per cent.
Owner:GENERAL RESEARCH INSTITUTE FOR NONFERROUS METALS BEIJNG
Method for synthesizing sodium aluminum hydride by utilizing Grignard reagent method
The invention discloses a method for synthesizing sodium aluminum hydride by utilizing a Grignard reagent method and relates to a method for preparing sodium aluminum hydride. The method for synthesizing sodium aluminum hydride by utilizing the Grignard reagent method comprises the following steps: preparing an aluminum Grignard reagent by utilizing bromoethane and activated aluminum powder, further removing halogen atoms, and generating aluminum alkyl hydride. An organic aluminum compound is prepared by reacting activated aluminum and bromoethane, and on the basis that a mixture of aluminum alkyl bromide and aluminum alkyl hydride is prepared, high-purity sodium aluminum hydride is prepared by directly reacting a high-activity organic aluminum reagent and sodium hydride.
Owner:ZHONGBEI UNIV
Sodium alanate and rare earth-nickel base alloy composite hydrogen storage material and preparation thereof
InactiveCN101412495AGood low temperature reversible hydrogen storage performanceGood phase structure stabilityHydrogen productionHigh energyRare earth
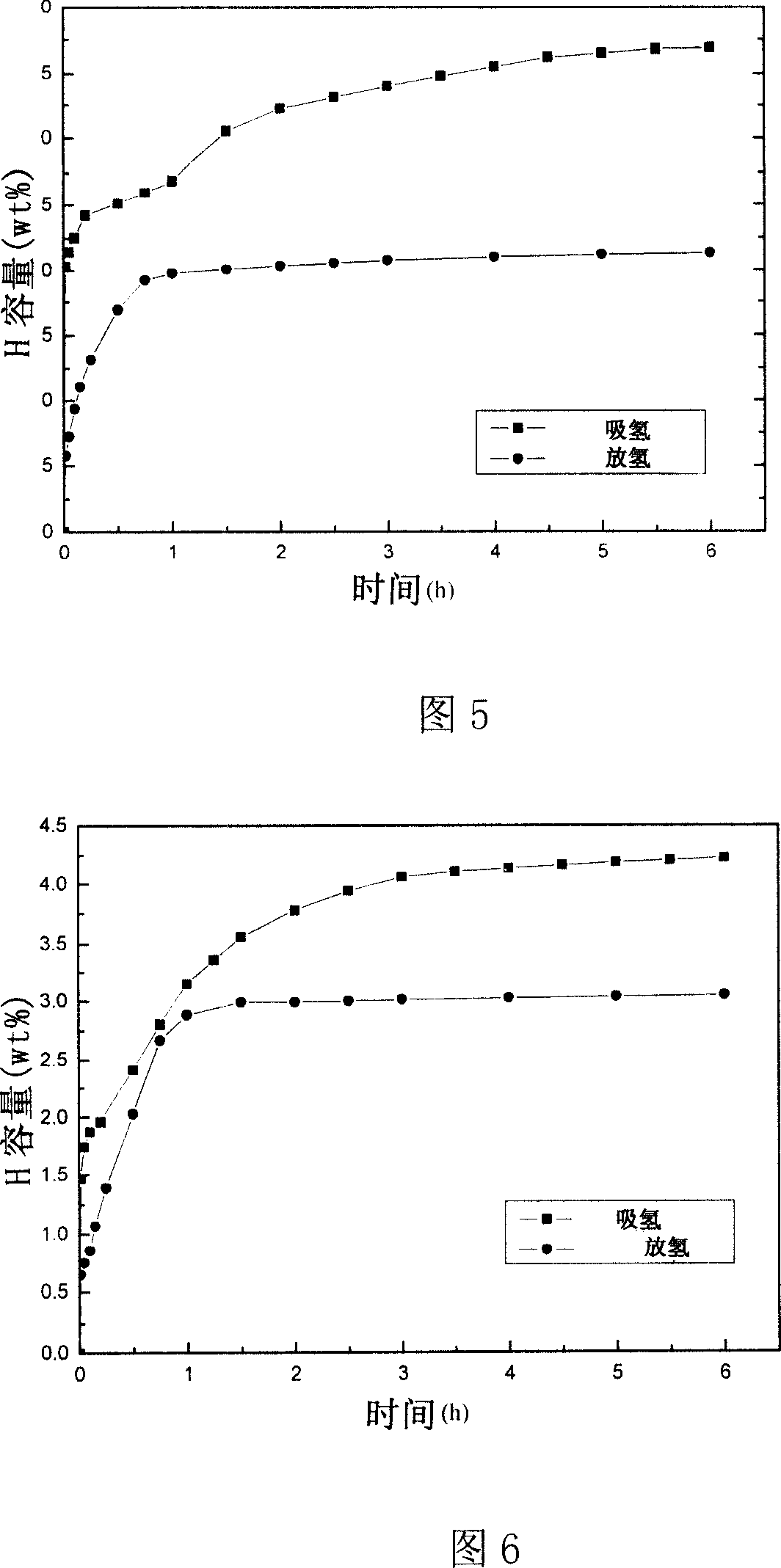
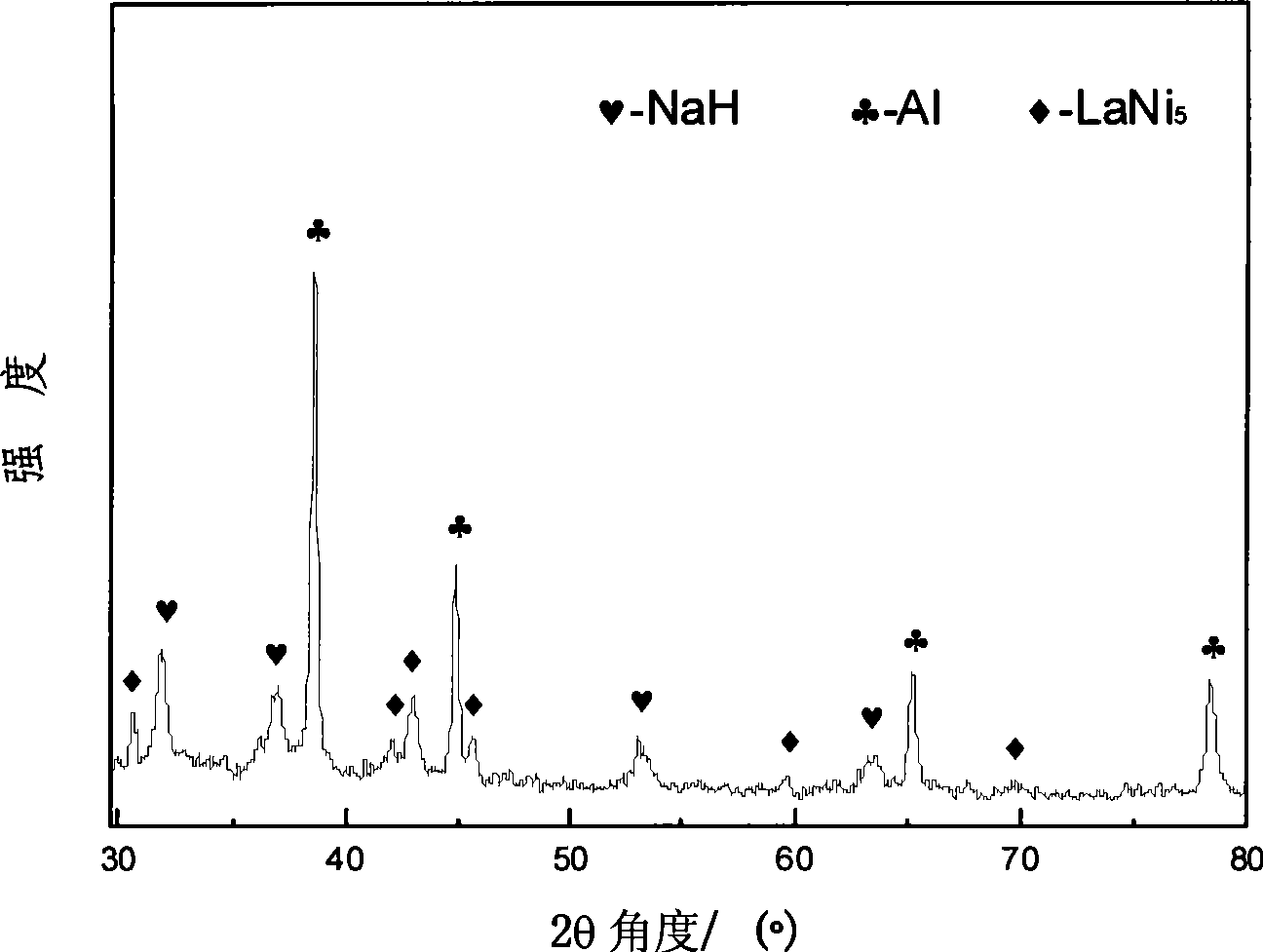
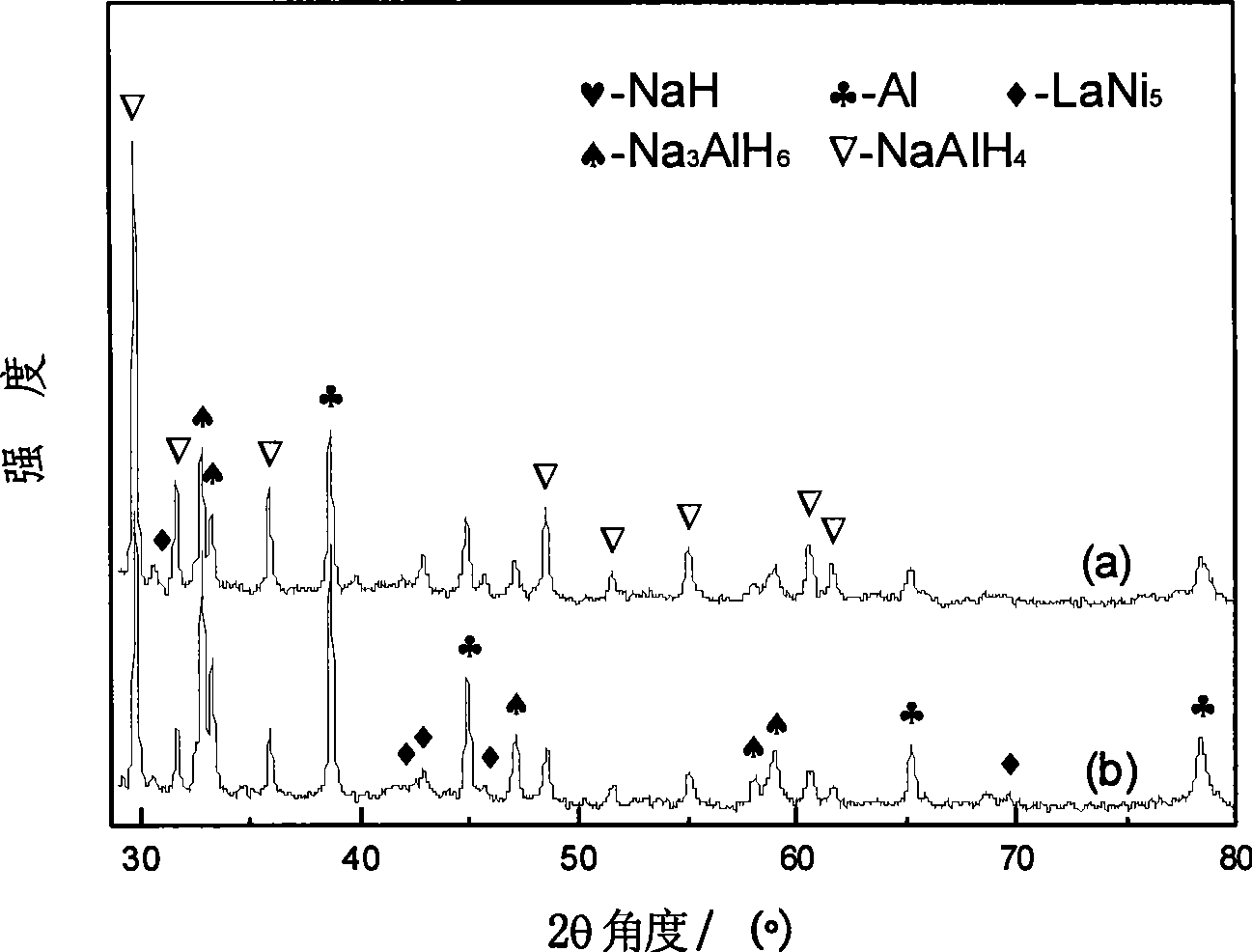
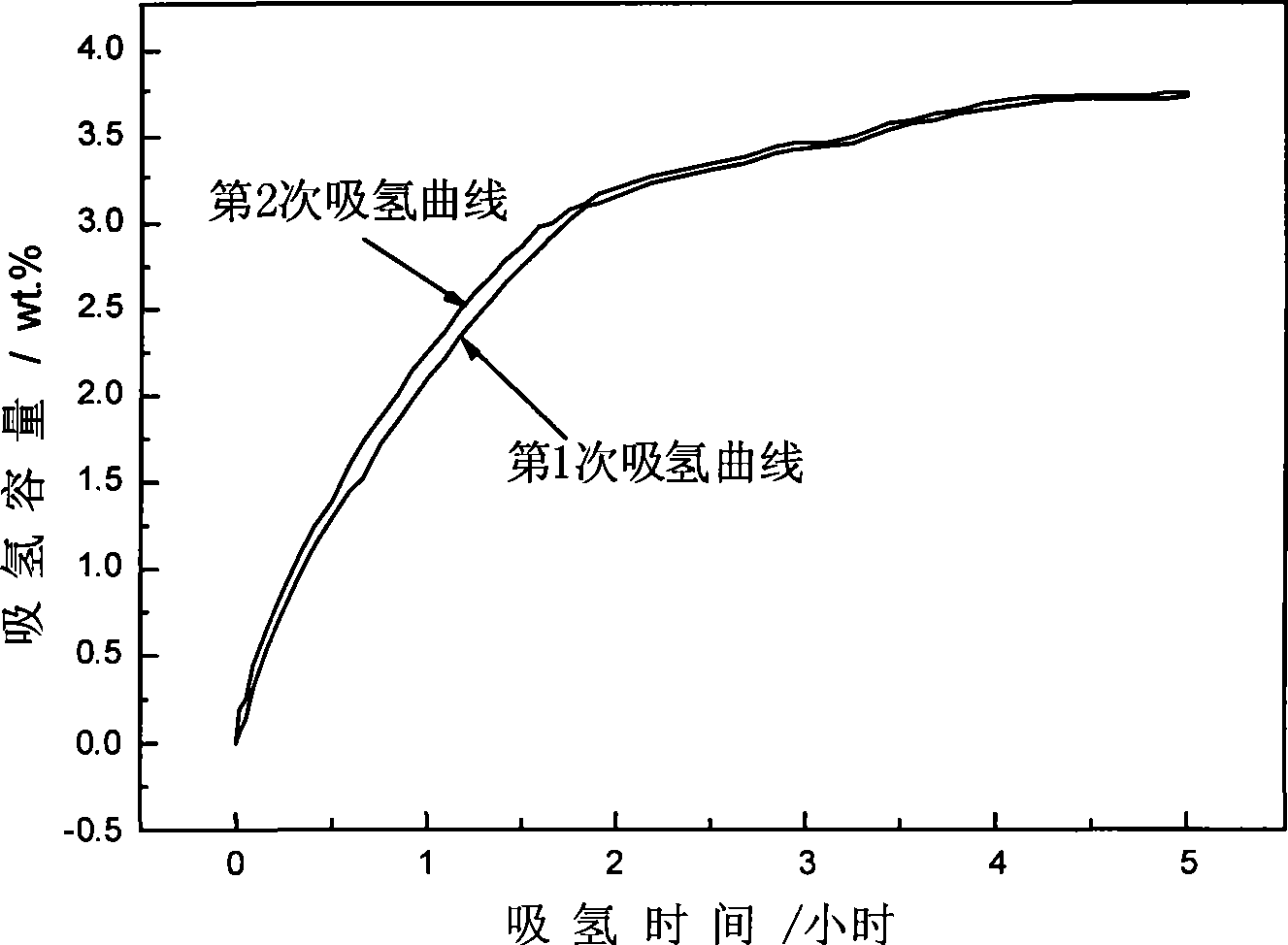
The invention discloses sodium aluminum hydride and rare earth-nickel base alloy composite hydrogen storage material consisting of sodium aluminum hydride and rare earth-nickel base alloy. The mass percent of the rare earth-nickel base alloy is between 5 and 35 percent and the balance being sodium aluminum hydride; wherein the rare earth-nickel base alloy has a chemical general expression of RENi5, and RE in the general expression can be La, Ce, Pr, Nd, Y, Ml or Mm. The preparation method for the sodium aluminum hydride and rare earth-nickel base alloy composite hydrogen storage material is a high-energy ball milling method. The preparation method is simple. The prepared sodium aluminum hydride and rare earth-nickel base alloy composite hydrogen storage material has good activation hydrogen storage performance and hydrogen discharging kinetics, and a reversible hydrogen storage capacity of above 3.7 weight percent without adding a special catalyst. The composite hydrogen storage material can be applied to miniature mobile phones, laptops, the hydrogen supply sources of independent galvanic pile systems, the field of hydrogen purification, and the like.
Owner:ZHEJIANG UNIV
Process for producing pyridine compound, and pyridine compound
Provided is a process for producing a high-purity pyridine compound from a crude pyridine compound that contains a diazine compound as an impurity, the method including a reaction step of reacting the crude pyridine compound with an aluminum hydride compound, and a distillation step of distilling the product obtained from the reaction step. The aluminum hydride compound preferably contains one or more compounds selected from lithium aluminum hydride and sodium aluminum hydride.
Owner:AIR WATER INC
Lithium borohydride/ alkali metal aluminum hydride/calcium carbide composite hydrogen storage material and preparation method thereof
ActiveCN106517089ALow hydrogen release temperatureHigh hydrogen releaseReversible hydrogen uptakeHydrogen desorptionALUMINUM HYDRIDE
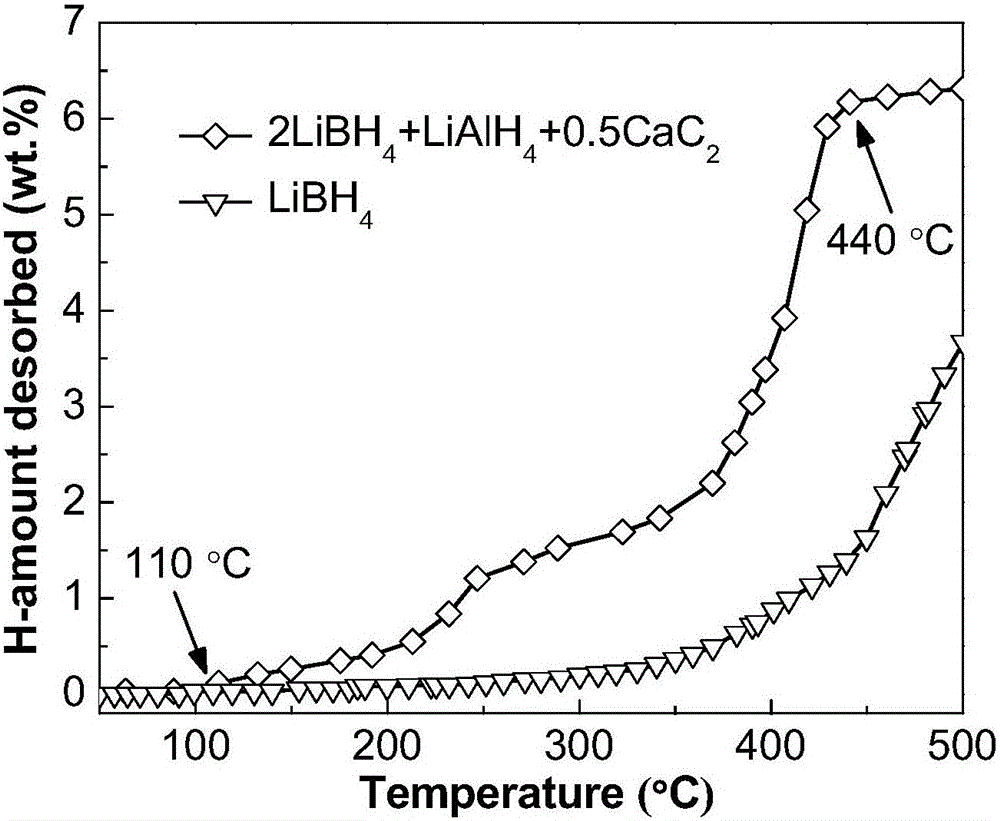
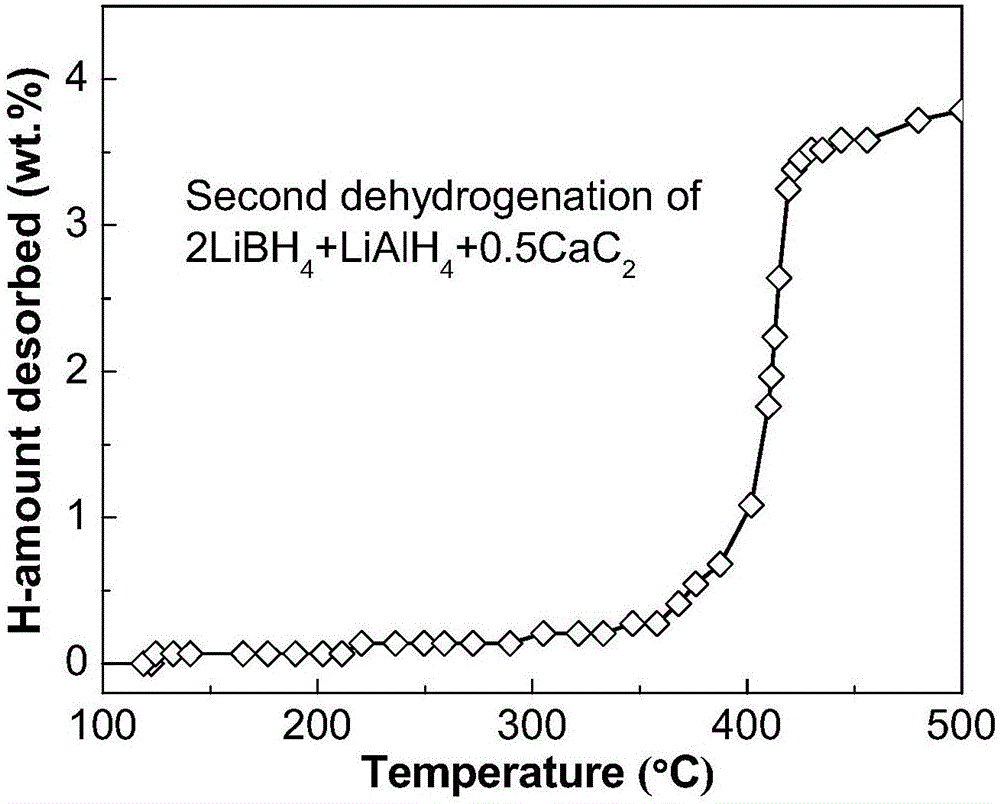
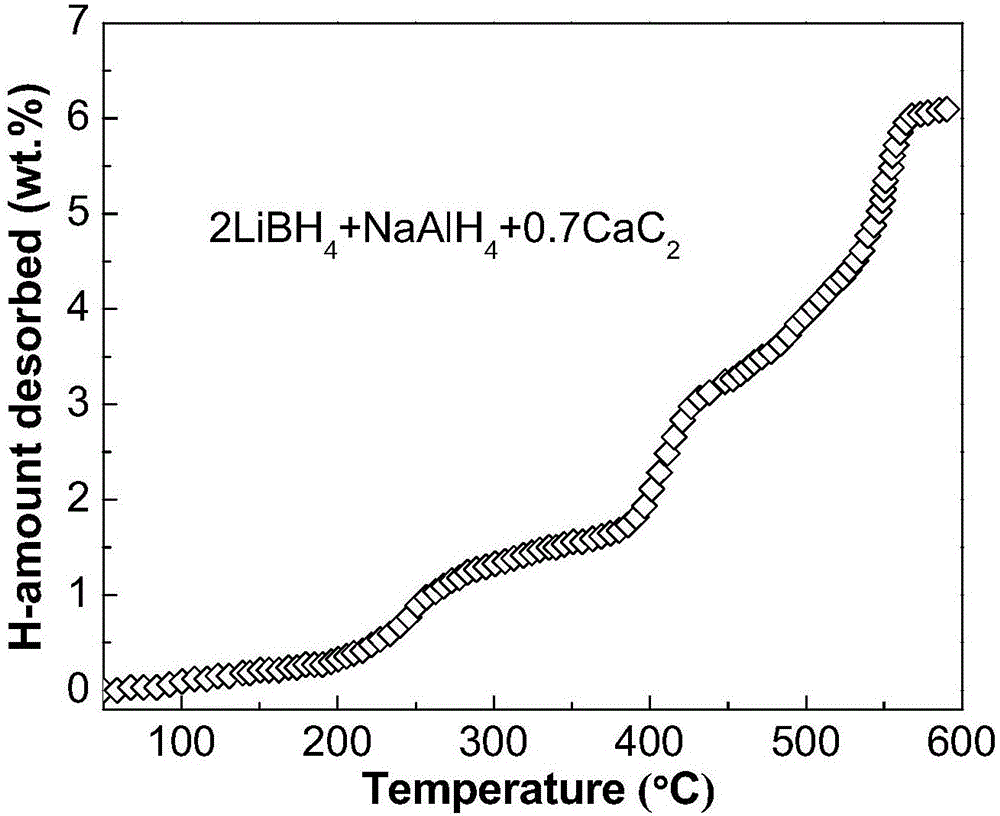
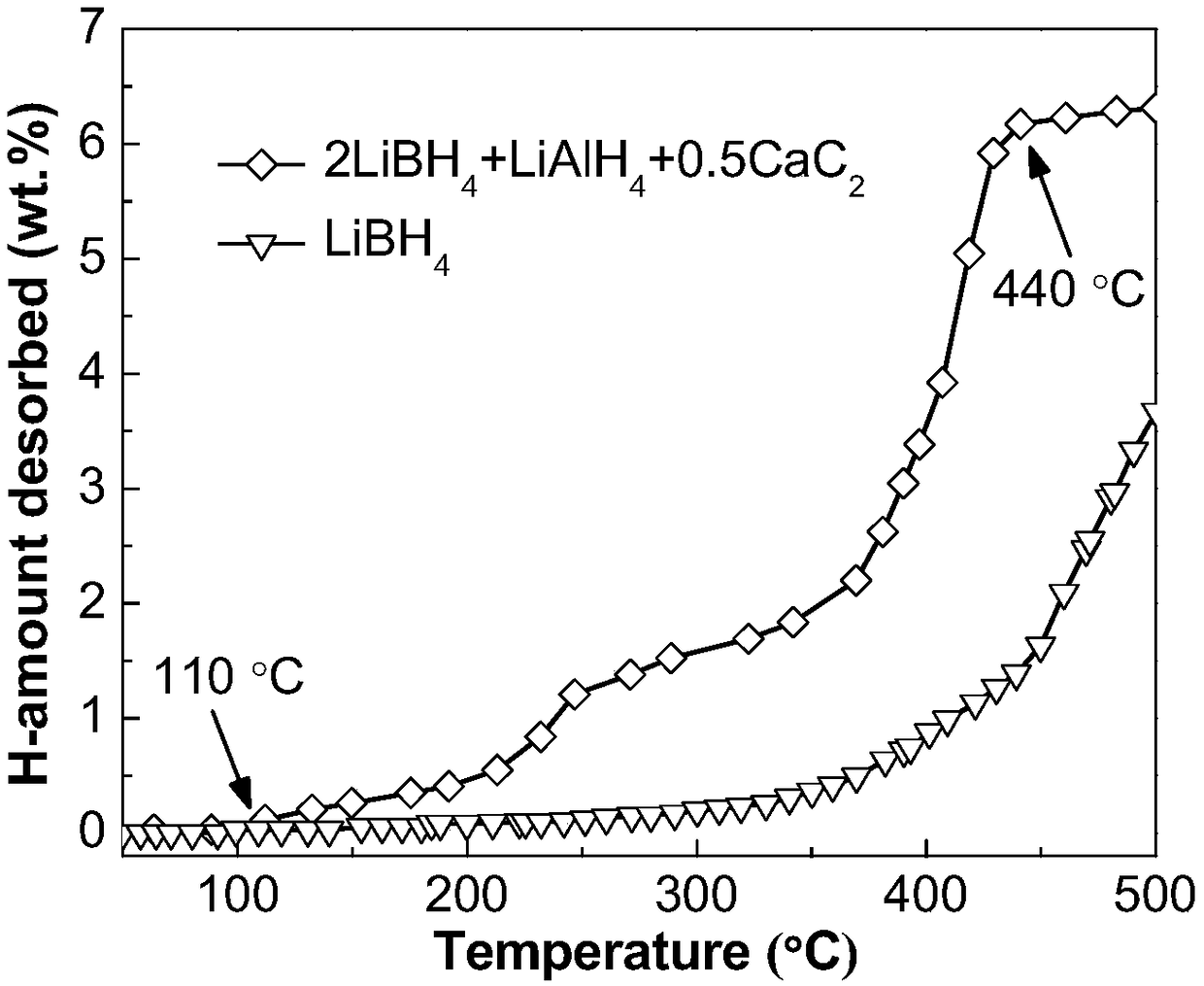
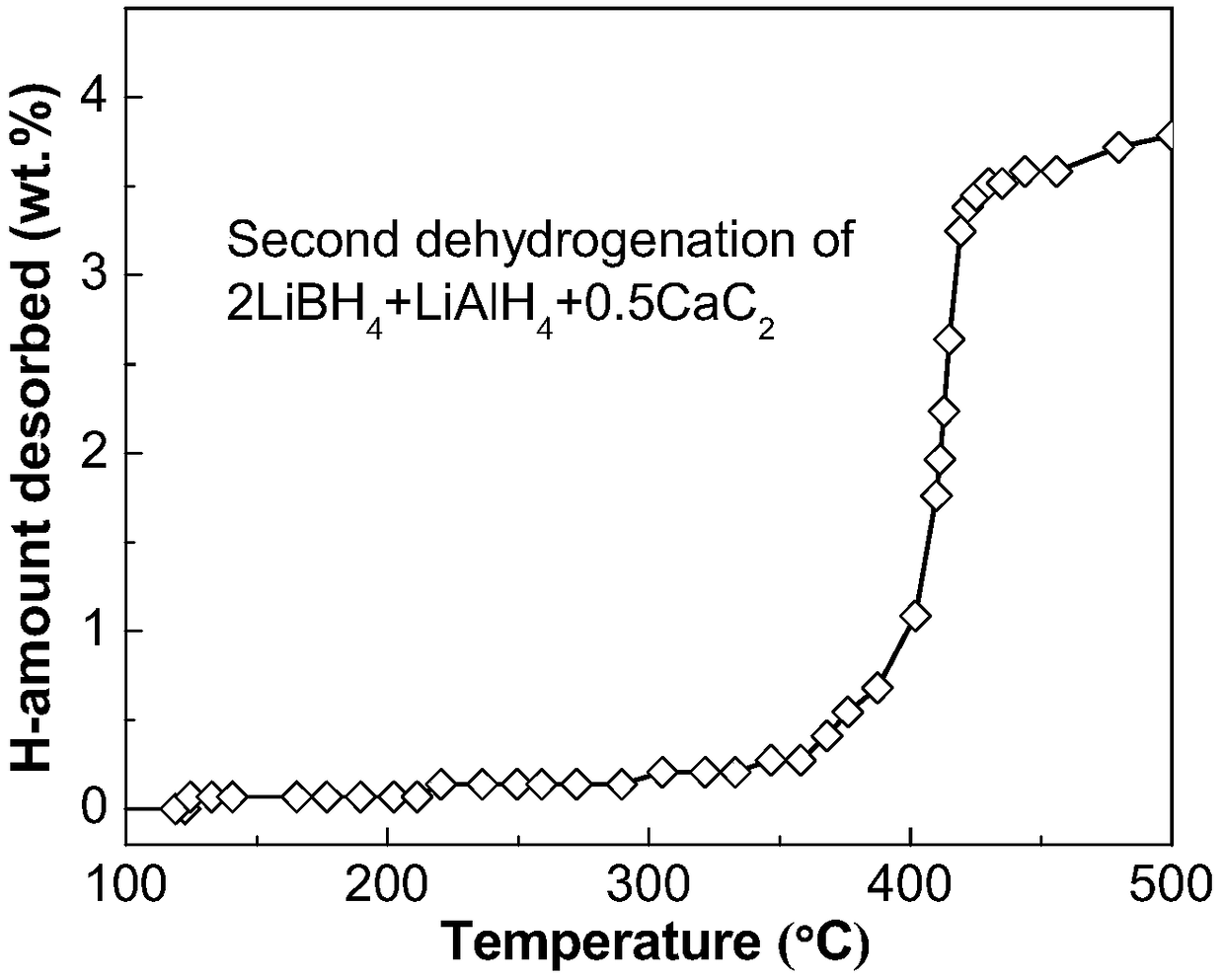
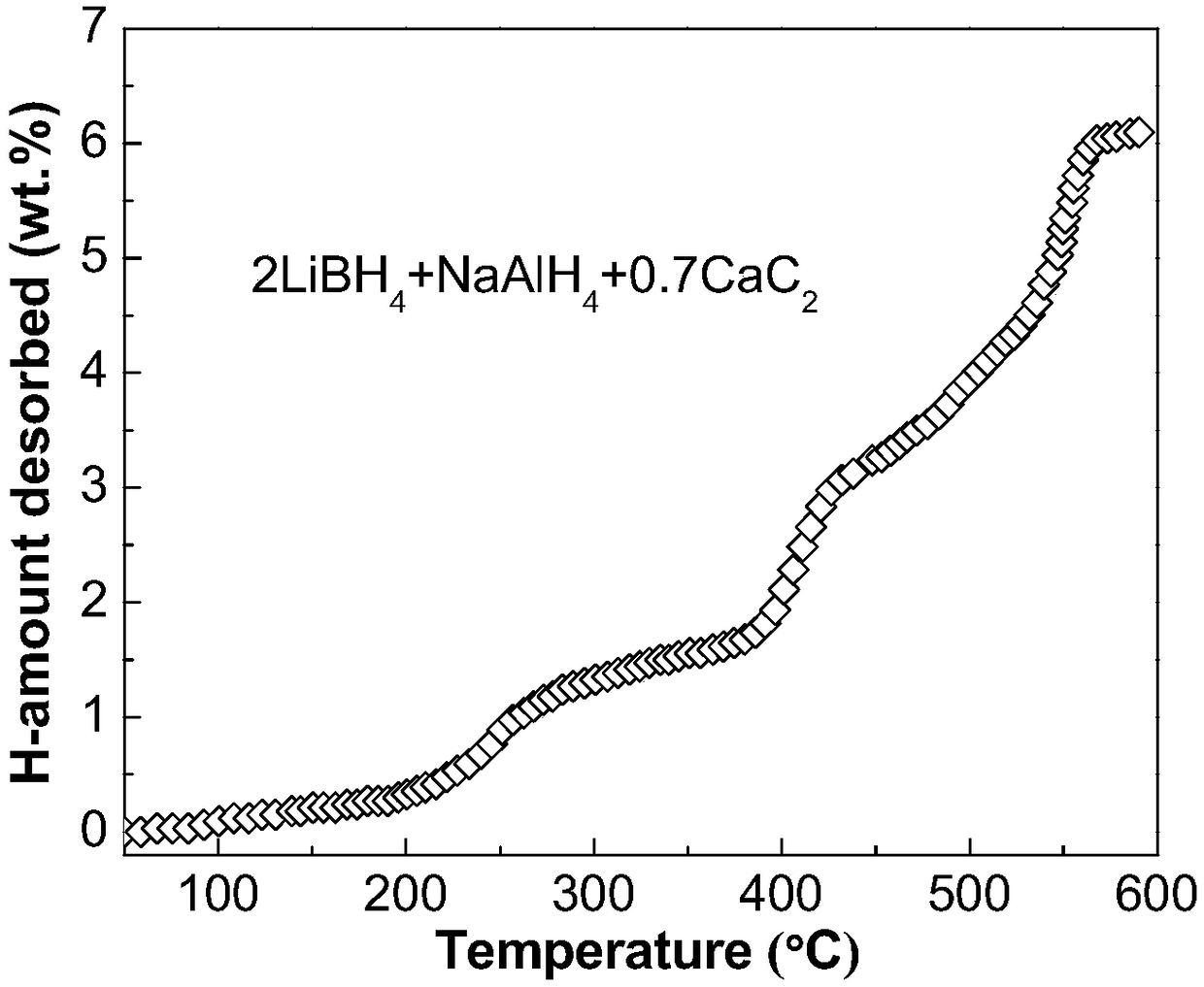
The invention discloses a lithium borohydride / alkali metal aluminum hydride / calcium carbide composite hydrogen storage material and a preparation method thereof and belongs to the technical field of hydrogen storage materials. The composite hydrogen storage material is prepared from lithium borohydride, alkali metal aluminum hydride and calcium carbide, wherein the mole ratio of lithium borohydride to alkali metal aluminum hydride is 2:1, the additive amount of calcium carbide is 12-25mol%, and alkali metal aluminum hydride is lithium aluminum hydride or sodium aluminum hydride. During preparation, calcium carbide with the purity not lower than 97% is ground into powder with the granularity smaller than 500 mu m, lithium borohydride, alkali metal aluminum hydride and the calcium carbide powder are weighed in proportion and mixed, and finally, the mixed powder is subjected to ball-milling treatment through a planetary ball mill. The composite hydrogen storage material has the advantages as follows: a preparation process of the composite hydrogen storage material is simple, safe and reliable, the composite hydrogen storage material has low hydrogen desorption temperature, high hydrogen desorption and good reversible hydrogen reabsorption performance, the hydrogen storage performance of the material is improved through calcium carbide, raw materials are widely sourced, and the cost is low.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Method and combined solution for increasing productivity of low-porosity, low-permeability and low-pressure reservoir oil well
InactiveCN105156082AHigh porosityImprove permeabilityFluid removalDrilling compositionPorosityChemical reaction
The invention provides a method and a combined solution for increasing productivity of a low-porosity, low-permeability and low-pressure reservoir oil well and relates to oil production technology. The method specifically includes: according to the hole volume of an operating well, calculating using quantity and proportion of a component A solution and a component B solution which are needed, and preparing the component A solution and the component B solution; sequentially adding the component A solution and the component B solution into the oil well for chemical reaction to increase productivity of the oil well, wherein the component A solution comprises nitric acid-alcohol complex, hydrazine, ammonium nitrate and water, and the component B solution comprises hydrazine nitrate, tetrachloroethylene, sodium hydride and sodium aluminum hydride. Physical and chemical effect, on a near-wellbore area of the oil well, of the chemical reaction between working solutions and between the working solutions and rock strata can be utilized to increase porosity, permeability, kinetic energy, heat energy and fluidity of a production layer so as to increase productivity of the oil well. The method integrates comprehensive effect, on oil-gas reservoirs, of physical and chemical processes and is simple in technique, controllable in process, low in cost and high in efficiency.
Owner:YANAN SHUANGFENG PETROLEUM TECH CO LTD
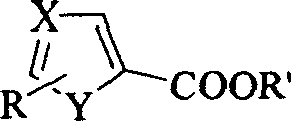
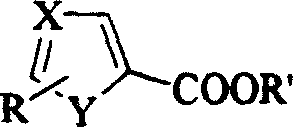
Heterocyclic carboxylic ester reducing process
The present invention relates to the method of reducing aromatic heterocyclic carboxylic ester to form alcohol. Aromatic heterocyclic carboxylic ester 5-methylol thiazole and 3-methylol pyridine are reduced with bis(2-methoxyoxethyl) sodium aluminum hydride as the reductant to obtain corresponding alcohol. Bis(2-methoxyoxethyl) sodium aluminum hydride has excellent dissolvability and this makes it possible to select solvent according to the reduced matter. Bis(2-methoxyoxethyl) sodium aluminum hydride has excellent heat stability, is stable at temperature up to 140 deg.c, will not burn spontaneously in the air and thus is safe. Bis(2-methoxyoxethyl) sodium aluminum hydride and the reduced matter 5- thiazolyl ethyl formate and 3-pyridyl ethyl formate can be dissolved well in arene, cheaper than tetrahydrofuran and easier in separation.
Owner:XIAMEN UNIV
Method for preparing alpha-aluminum trihydride through electrochemical catalytic deposition
InactiveCN106995926AEffective content regulationHigh purityElectrolysis componentsElectrode potentialHigh energy
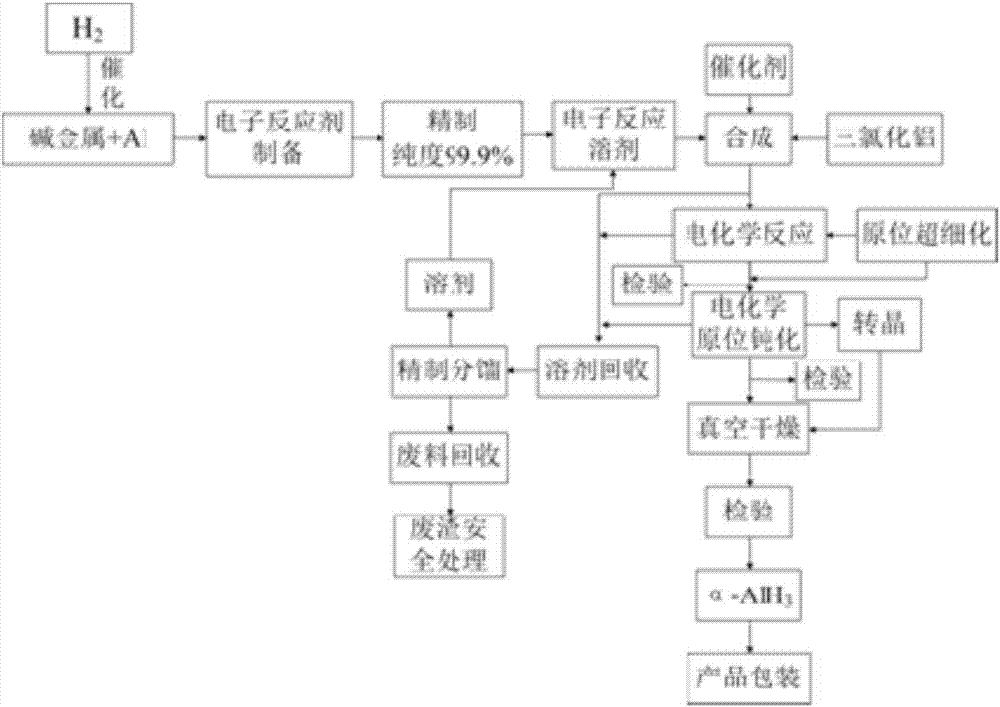
The invention discloses a method for preparing alpha-aluminum trihydride through electrochemical catalytic deposition. The method comprises the following step: (1) refining sodium aluminum hydride; (2) preparing alpha-aluminum trihydride through electrochemical catalytic deposition; and (3) post-treating the product. The method for preparing alpha-aluminum trihydride through electrochemical catalytic deposition is simple to operate, high in efficiency, free from chemical pollution, and small in energy consumption, so that the problems such as high energy consumption and high pollution in etherate and ion exchange method for preparing alpha-AlH3 can be solved. The method is safe, reliable, and high in yield; the electrochemical catalytic deposition reaction is carried out, so that toxic or dangerous oxidants and reducing agents are avoided; the product yield and the available content of the alpha-aluminum trihydride can be realized through electrode potential control; the product yield is up to 64-70%, and the available content of alpha-aluminum trihydride is up to be more than 98%. Therefore, the yield and the selectivity are high.
Owner:河南纳宇新材料有限公司
High-capacity hydrogen-storage material with NaAlH4 and preparation method thereof
InactiveCN1843614ASimple processEasy to operateOther chemical processesAlkaline earth metalStorage material
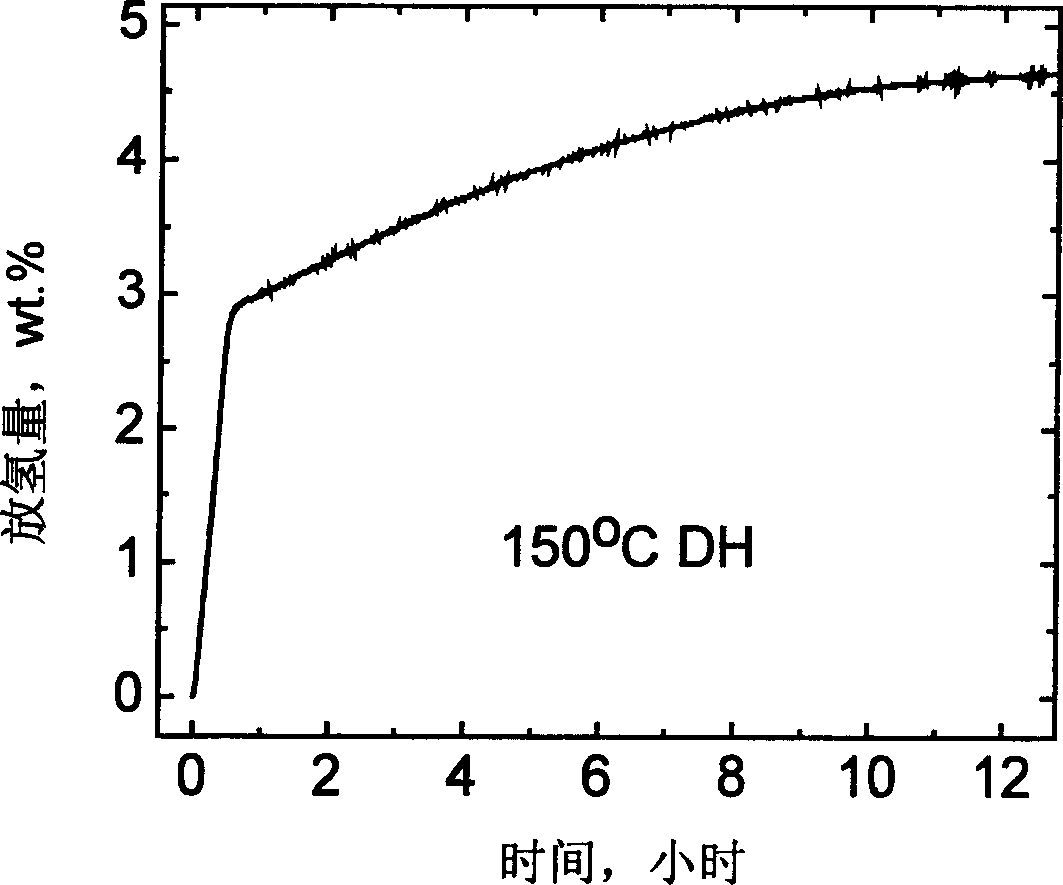
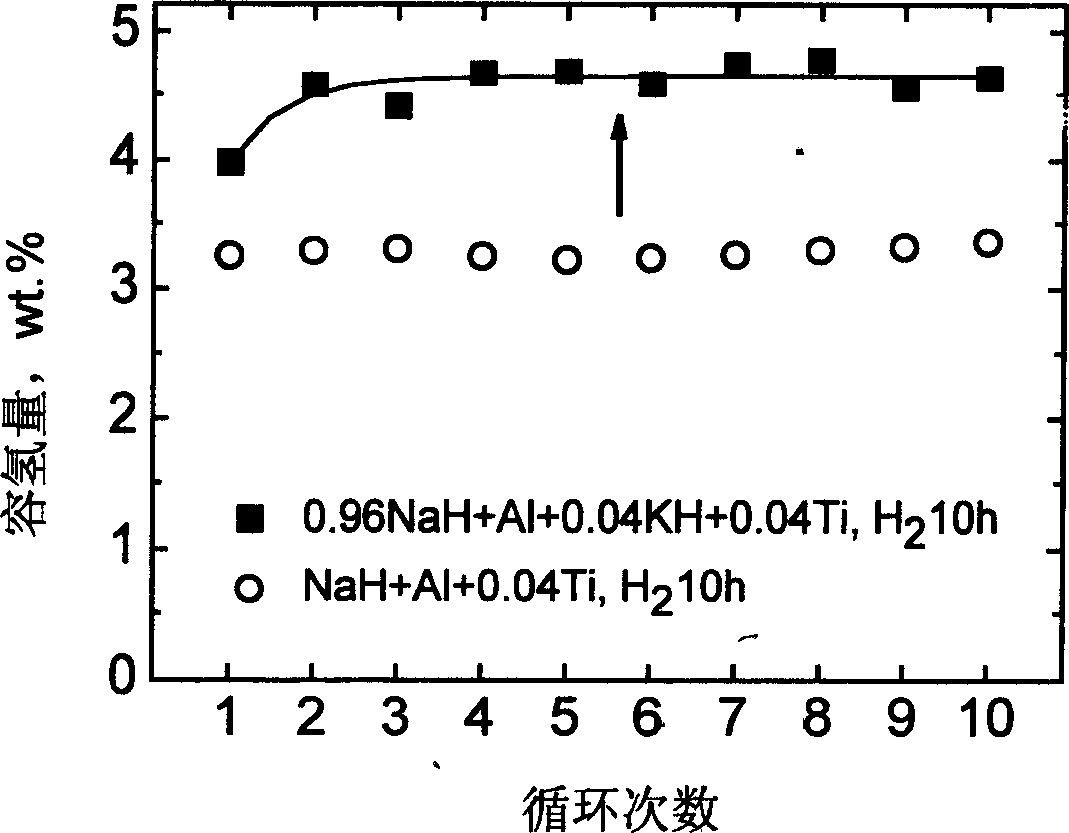
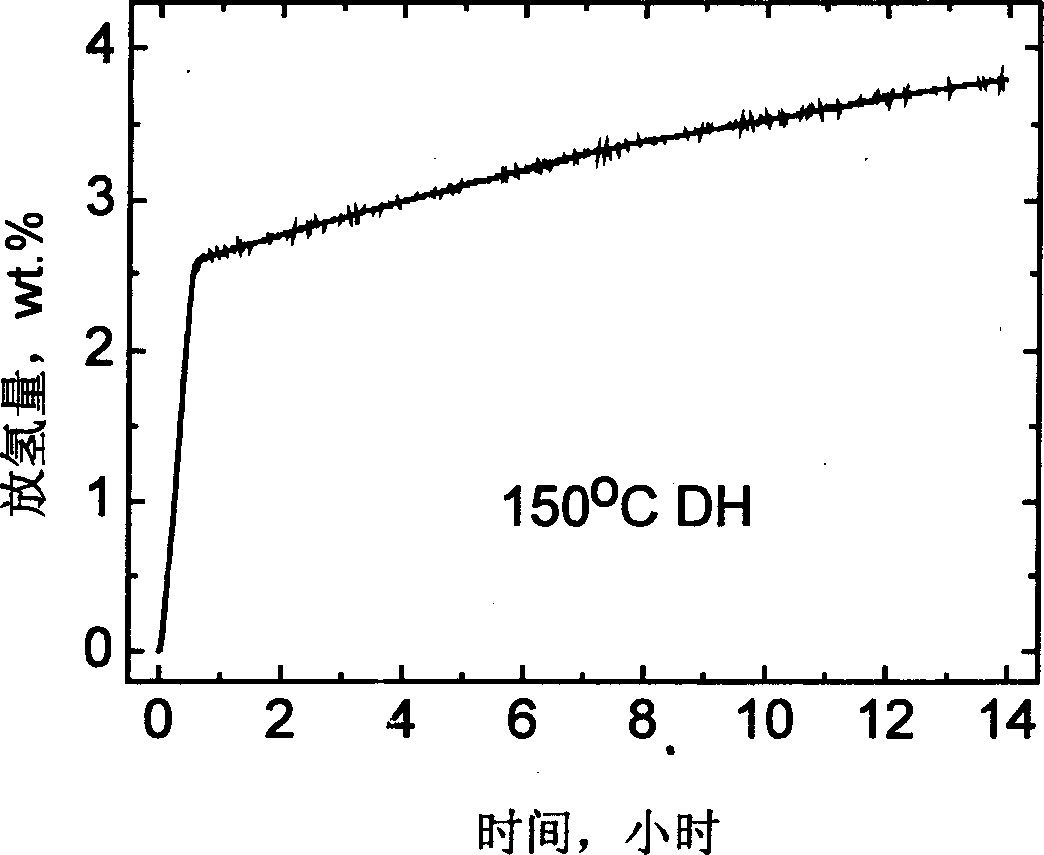
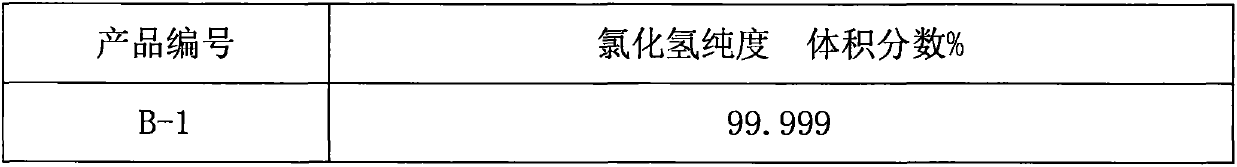
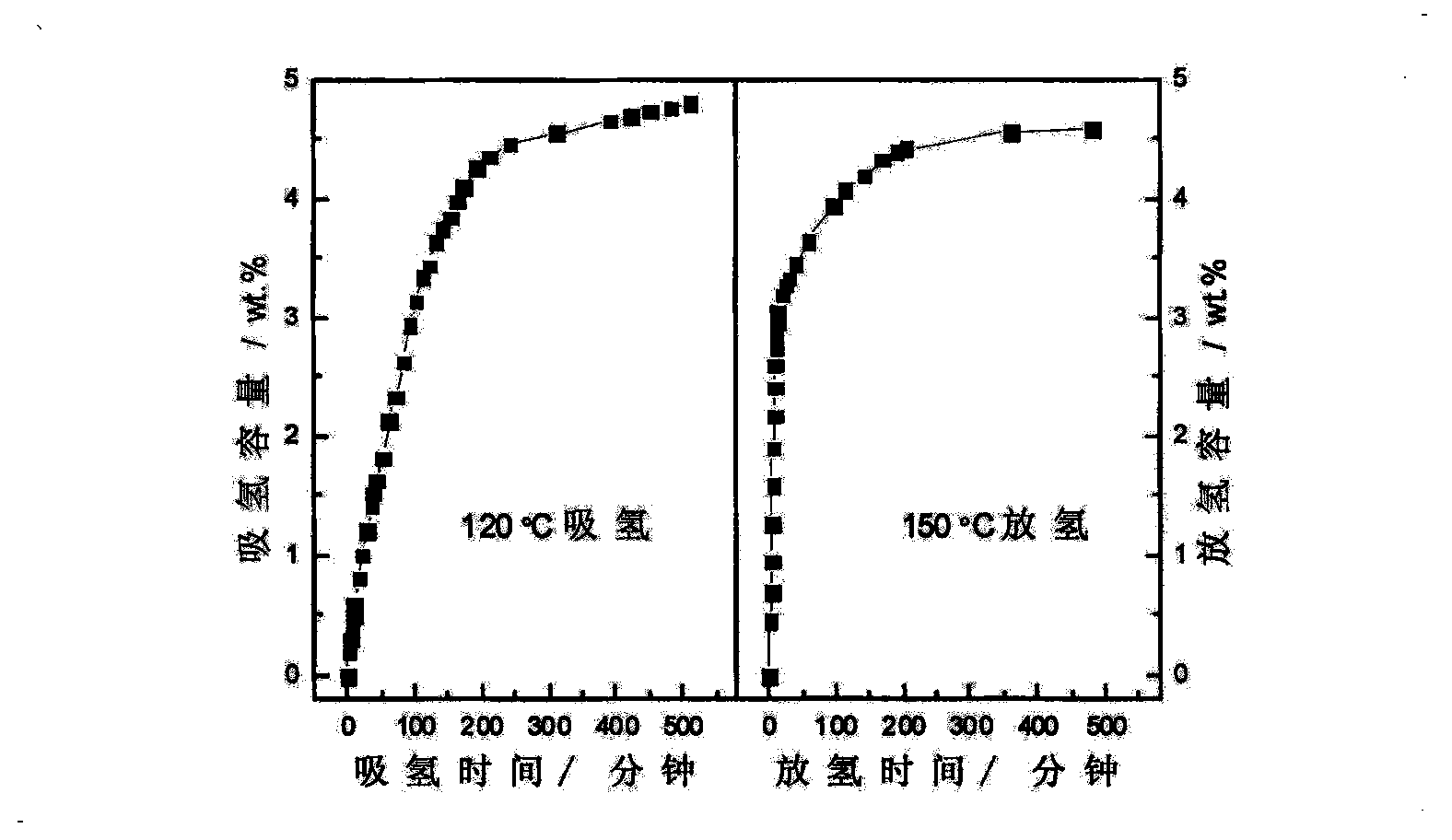
The invention relates to an improvement on the hydrogen storage material, especially providing a high-capacity coordinated sodium aluminum hydride storage material which is impure with transition metal. Said hydrogen system is formed by basic material, catalyst, and auxiliary structure. In the doping process, it directly uses transition metal as catalyst, and adding alkali metal / alkali hydride or alkaline earth metal / alkaline earth hydride as auxiliary structure at the same time; and processing ball grinding under inertia gas protection or reactive hydrogen gas. The invention has the advantages that: the method is simple, with easy operation and lower cost, while it can solve the problems of traditional techniques which will generate inertia by-product and foreign gas; and provided system has high capacity of hydrogen and high cycle stability. The actual hydrogen capacity can reach 4.7wt% in some system which is increased 40% than present ones.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Hydrogenation heat gas chemical yield increasing solution component applied to shallow well
ActiveCN102942913BHigh porosityImprove permeabilityFluid removalDrilling compositionPorosityAmmonium nitrate
The invention belongs to the technical field of oil exploitation, and relates to a hydrogenation heat gas chemical yield increasing solution component capable of increasing permeability of a shallow well and a near wellbore region. The solution component comprises first solution and second solution, the mass ratio of the first solution to the second solution is 1:1, the first solution comprises 37-43% of ammonium nitrate NH4NO3, 23-26% of urea CO(NH2)2, 5-8% of decaborane B10H14, 1-2% of glucose C6H12O6, 0.5-1.5% of methenamine (CH2)6N4 and 19.5-33.5% of water. The second solution comprises 44-48% of sodium nitrate NaNO3, 15-20% of sodium nitrite NaNO2, 17-21% of sodium aluminum hydride NaAlH4 and 15-19% of tetrachloroethylene C2Cl4. By the aid of the solution component, porosity of an ore bed can be increased, accordingly, permeability is increased, and the yield can be increased by 2-10 times.
Owner:吉林贯通能源科技有限责任公司
Florescent silicon oxide nanoparticle preparation method
InactiveCN106118636AExcellent fluorescence performanceOperational securityHydrocarbon purification/separationNanoopticsSilicon oxideFluorescein isothiocyanate
The invention discloses a method for preparing fluorescent silicon oxide nanoparticles, which belongs to the technical field of silicon oxide particles. In the present invention, quartz sand is used as raw material to make silicon dioxide through nanometerization to make nano-silicon dioxide, and then the prepared nano-silicon dioxide and hydrofluoric acid form silicon fluoride, and then react with sodium aluminum hydride , to produce silane gas, and then use lycopene and fluorescein isothiocyanate to vaporize and mix with silane gas, add hydrogen peroxide and place it in a strong magnetic field for reaction, so as to obtain fluorescent silicon oxide nanoparticles. Examples prove that the present invention is safe to operate. By gasifying lycopene and fluorescein isothiocyanate extracted from tomatoes, it is environmentally friendly and at the same time enhances the fluorescence of fluorescent silicon oxide nanoparticles. In addition, hydrogen peroxide is added and placed in a strong magnetic field reaction, forming free radicals to generate corona, improving stability, and the prepared fluorescent silica nanoparticles have extremely narrow half-width of fluorescence spectrum, strong luminosity, and can be applied on a large scale.
Owner:高大元
Process for preparing alcohol by heterocyclic carboxylic ester
Owner:XIAMEN UNIV
Sodium alanate and rare earth-nickel base alloy composite hydrogen storage material and preparation thereof
InactiveCN101412495BComposite uniformSmall particle sizeHydrogen productionRare earthAlloy composite
The invention discloses sodium aluminum hydride and rare earth-nickel base alloy composite hydrogen storage material consisting of sodium aluminum hydride and rare earth-nickel base alloy. The mass percent of the rare earth-nickel base alloy is between 5 and 35 percent and the balance being sodium aluminum hydride; wherein the rare earth-nickel base alloy has a chemical general expression of RENi5, and RE in the general expression can be La, Ce, Pr, Nd, Y, Ml or Mm. The preparation method for the sodium aluminum hydride and rare earth-nickel base alloy composite hydrogen storage material is ahigh-energy ball milling method. The preparation method is simple. The prepared sodium aluminum hydride and rare earth-nickel base alloy composite hydrogen storage material has good activation hydrogen storage performance and hydrogen discharging kinetics, and a reversible hydrogen storage capacity of above 3.7 weight percent without adding a special catalyst. The composite hydrogen storage material can be applied to miniature mobile phones, laptops, the hydrogen supply sources of independent galvanic pile systems, the field of hydrogen purification, and the like.
Owner:ZHEJIANG UNIV
Method for preparation of lithium aluminum hydride from sodium aluminum hydride
InactiveUS20030003045A1Increase concentrationAlkali/alkaline-earth/beryllium/magnesium hydridesMultiple metal hydridesLithium chlorideSodium aluminium hydride
A method for preparing a lithium aluminum hydride solution comprising lithium aluminum hydride, toluene and tetrahydrofuran by the steps of: (a) combining lithium chloride, tetrahydrofuran, and a slurry of sodium aluminum hydride in toluene; and (b) allowing the mixture formed in step (a) to react to form a product mixture comprising lithium aluminum hydride, sodium chloride, tetrahydrofuran and toluene.
Owner:ROHM & HAAS CO
Hydrogenation heat gas chemical yield increasing solution component for low-permeability sandstone reservoir oil well
ActiveCN102942912BRealization of production increaseHigh porosityFluid removalDrilling compositionMass ratioHigh pressure
The invention belongs to the technical field of oil exploitation, and particularly relates to a hydrogenation heat gas chemical yield increasing solution component capable of being used for increasing productivity of a low-permeability sandstone reservoir oil well. The solution component comprises first solution and second solution, the mass ratio of the first solution to the second solution is 1:1, by the mass sum of 100%, the first solution comprises nitric acid cholamine complex HOCH2CH2NH2*HNO3, ammonium nitrate NH4NO3 and water H2O, and the second solution comprises hydrazine nitrate N2H4*HNO3, sodium hydride NaH, sodium aluminum hydride NaAlH4 and tetrachloroethylene C2Cl4. Gas released in reaction of the first solution and the second solution can carry heat and enter air holes and micro-cracks of near wellbore region rock strata through a well-cased perforating area, the near wellbore region rock strata can generate new cracks under continuous heat shock action of high-temperature high-pressure gas, oil-gas seepage channels are communicated, seepage resistance is decreased, drainage area is increased, and accordingly, permeability of the near wellbore region rock strata is improved.
Owner:吉林贯通能源科技有限责任公司
Method for reduction of substituted malonates to diols
InactiveUS6538163B2Organic compound preparationOxygen compounds preparation by reductionDiolMalonate
A method for reducing a malonate having the formula R1R2C(CO2R3)(CO2R4) to a diol having the formula R1R2C(CH2OH)2 comprising treating said malonate with sodium aluminum hydride.
Owner:ROHM & HAAS CO
Method for reduction of substituted malonates to diols
InactiveUS20030036666A1Organic compound preparationOxygen compounds preparation by reductionDiolMalonate
A method for reducing a malonate having the formula R1R2C(CO2R3)(Co2R4) to a diol having the formula R1R2C(CH2OH)2 comprising treating said malonate with sodium aluminum hydride.
Owner:ROHM & HAAS CO
Dehydrating method for organic solvent
ActiveCN104151146AGood effectSuitable for dehydration productionEther separation/purificationHydrocarbon purification/separationChemical reactionOrganosolv
The invention discloses a dehydrating method for an organic solvent. In the method, a chemical reaction method is adopted. The method comprises the following steps: reacting a small amount of water in the organic solvent with a sodium aluminum hydride solution; transforming all the water in the organic solvent into harmless solid slurry; reacting the excessive sodium aluminum hydride with silicon tetrafluoride; and distilling and drying a reaction product to obtain an organic solvent of which the water content is less than 300ppm. By adopting the method, the problem that organic solvents produced in chemical production are polluted by water is solved completely. The dehydrating method has the advantages of low cost, easiness in operation, suitability for dehydrating production of organic solvents in chemical enterprises, and the like.
Owner:ZHEJIANG ZHONGNING SILICON IND
High-capacity hydrogen-storage material with NaAlH4 and preparation method thereof
InactiveCN100369665CSimple processEasy to operateOther chemical processesAlkaline earth metalPtru catalyst
The invention relates to an improvement on the hydrogen storage material, especially providing a high-capacity coordinated sodium aluminum hydride storage material which is impure with transition metal. Said hydrogen system is formed by basic material, catalyst, and auxiliary structure. In the doping process, it directly uses transition metal as catalyst, and adding alkali metal / alkali hydride or alkaline earth metal / alkaline earth hydride as auxiliary structure at the same time; and processing ball grinding under inertia gas protection or reactive hydrogen gas. The invention has the advantages that: the method is simple, with easy operation and lower cost, while it can solve the problems of traditional techniques which will generate inertia by-product and foreign gas; and provided system has high capacity of hydrogen and high cycle stability. The actual hydrogen capacity can reach 4.7wt% in some system which is increased 40% than present ones.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Preparation method of silane
InactiveCN104445209ALow costDecomposition reaction conditions are mildSilicon hydridesHydrogen fluorideSilanes
The invention provides a preparation method of silane. The method comprises steps of albite decomposition reaction, silicon fluoride purification and silane preparation, wherein the albite decomposition reaction comprises steps as follows: mixing albite, fluorite and sulfuric acid with concentration of 98% at a mass ratio of 1: (1.55-2.57): (3.28-4.62) and performing reaction at the temperature of 100-200 DEG C under the self-generated pressure to obtain solid residues and silicon fluoride contained gas; the silicon fluoride purification comprises steps as follows: performing dust removal on the silicon fluoride contained gas, liquefying the silicon fluoride contained gas after dust removal to obtain a silicon fluoride liquid, removing hydrogen fluoride in the silicon fluoride liquid to obtain a silicon fluoride liquid free of hydrogen fluoride, and gasifying the silicon fluoride liquid free of hydrogen fluoride to obtain pure silicon fluoride gas; and silane preparation comprises step as follows: introducing the pure silicon fluoride gas into a sodium aluminum hydride solution for reaction to obtain a silane product. According to the preparation method, gas products including silicon fluoride and sodium aluminum hydride which are generated after albite decomposition are mainly utilized and react to prepare silane, and one novel silane preparation method capable of realizing continuous production is provided.
Owner:HENAN ZHILIAN HUANYU INTPROP OPERATION CO LTD
A kind of purification method of electronic grade hydrogen chloride
ActiveCN106044710BImprove the performance of hydrogen adsorptionImprove adsorption capacityChlorine/hydrogen-chloride purificationSorbentIon exchange
The invention relates to a method for purifying electron-grade hydrogen chloride. The method comprises the following steps: carrying out ion exchange between highly basic polystyrene macroporous ion exchange resin with sodium aluminum hydride NaAlH4 to generate an aluminum hydride resin adsorbent; loading 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, so that the performances of adsorbing hydrogen, carbon dioxide and other impurities are improved.
Owner:ZHEJIANG BRITECH CO LTD
Nano-catalyst of sodium aluminum hydride complex hydride as well as preparation method and application thereof
InactiveCN101406843BHigh activityImprove stabilityPhysical/chemical process catalystsMetal hydridesNano catalystPtru catalyst
The invention relates to a nanometer catalyst for aluminum sodium hydride complex hydrides, as well as a preparation method and application thereof. The chemical formula of the catalyst is TixC1-x, wherein x is between 0.2 and 0.8. A method for preparing the catalyst comprises the following steps: according to the stoichiometric ratio of TixC1-x, simple-substance Ti and C powder are mixed, cold-pressed, molded, sintered, diffused and sintered in argon, cooled with a furnace, ground and ball-milled in a vibrating ball mill so as to obtain TixC1-x catalyst ultrafine powder. When the catalyst isused for reversibly-stored hydrogen of aluminum sodium hydride, the catalyst has good catalytic performance and can improve the capacity of the reversibly-stored hydrogen of the aluminum sodium hydride by more than 4.5 percent in weight. The catalyst has the advantages of simple preparation process, low cost and good activity and stability.
Owner:ZHEJIANG UNIV
Method for producing polycrystalline silicon by utilizing sodium fluosilicate byproduct of phosphate fertilizer
ActiveCN102070144BReduce manufacturing costLow impurity contentSilicon hydridesSilicon tetrafluorideMaterials science
Owner:DO FLUORIDE CHEM CO LTD
A lithium borohydride/alkali metal aluminum hydride/calcium carbide composite hydrogen storage material and its preparation method
ActiveCN106517089BLow hydrogen release temperatureHigh hydrogen releaseReversible hydrogen uptakeHydrogen desorptionALUMINUM HYDRIDE
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com