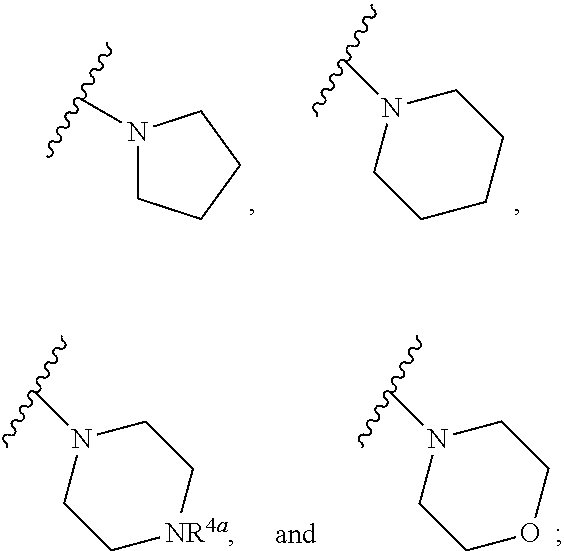
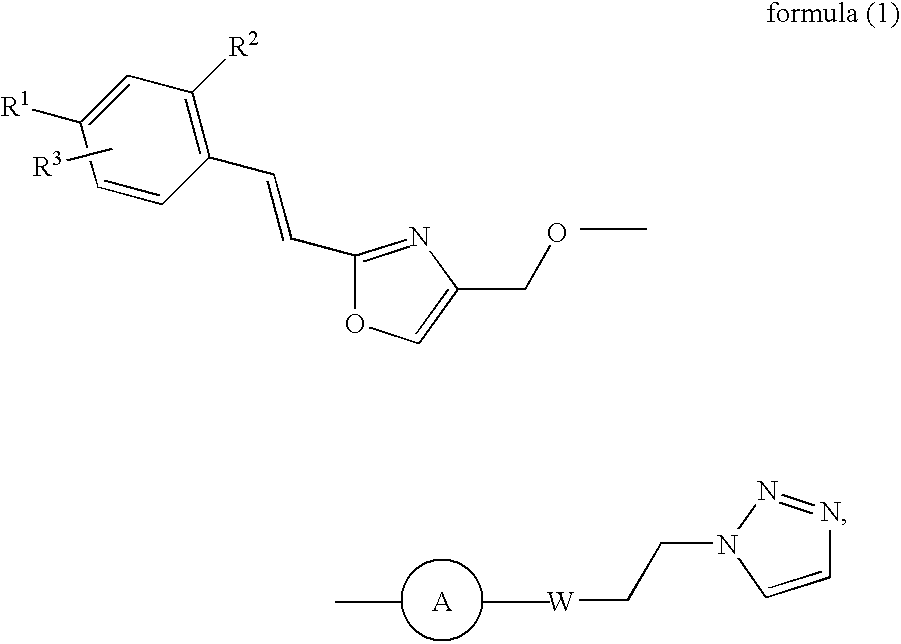
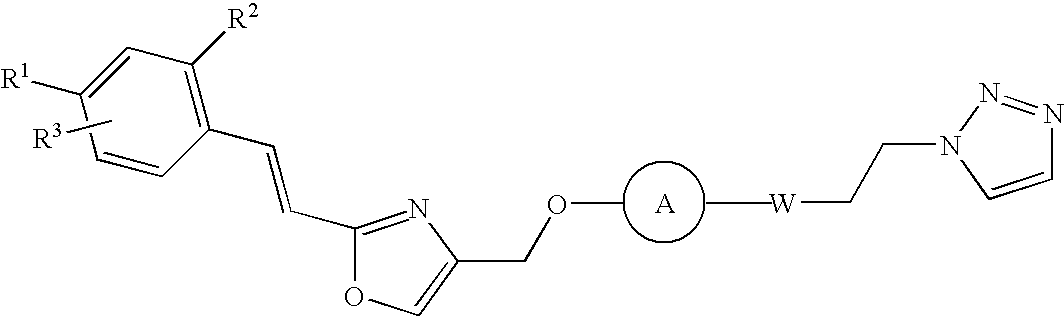
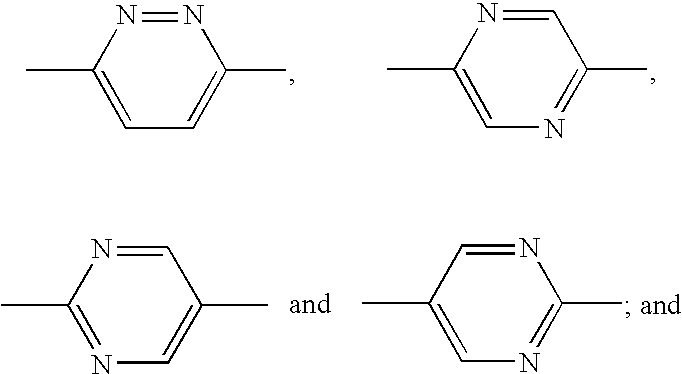
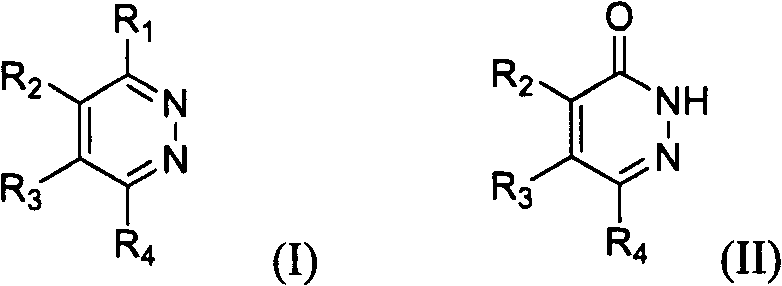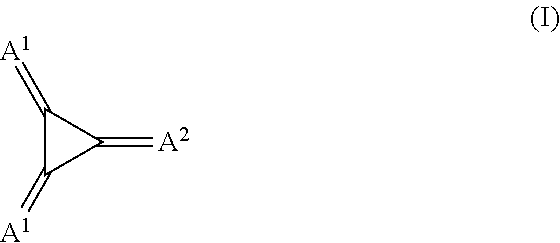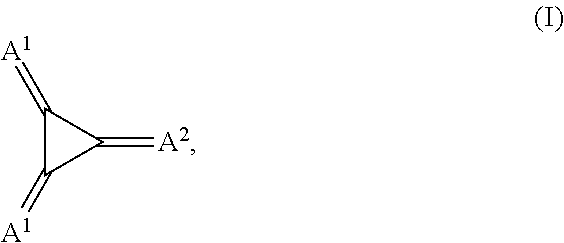Patents
Literature
71 results about "Diazine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
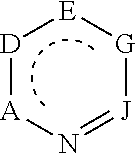
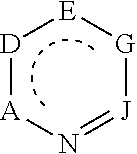
Diazines are a group of organic compounds having the molecular formula C₄H₄N₂. Each contains a benzene ring in which two of the C-H fragments have been replaced by isolobal nitrogen.
Phosphorescent dye parent materials with tetrahedral structure, and application thereof in electroluminescent device
InactiveCN102775432AImprove migration abilityHigh electroluminescence efficiencyGroup 4/14 element organic compoundsGroup 5/15 element organic compoundsDisplay deviceQuinoline
The invention belongs to the technical field of organic electroluminescence material, in particular to a series of phosphorescent dye parent materials with a tetrahedral structure, and the application of the materials in an electroluminescent device. The phosphorescent dye parent materials can be used for a panel display device, a light-emitting diode (LED) and an electronic imaging device. The phosphorescent dye parent material has excellent electronic and hole transmission properties, can be independently used as a luminous layer and a current carrier transmission layer, and can be used as matrix and doped with other fluorescent or phosphorescent dyes. The compound has stronger fluorescent property in a state of solution or solid, can be used for forming an even film, and has better optical stability and thermal stability. R1, R2, R3 and R4 are respectively connected with phenyl group by a single bond, and are respectively selected from one of hydrogen, alkyl group, alkoxy, nitryl, hydroxyl, cyano-group, benzene cyano-group, amino, sulfydryl, halogen, diphenyl phosphoryl, furan, thiophene, pyrrole, pyridina, diazine, triazine, pyran, quinoline, benzpyrole, carbazole, aniline or phenothiazine, and X is C or Si.
Owner:JILIN UNIV
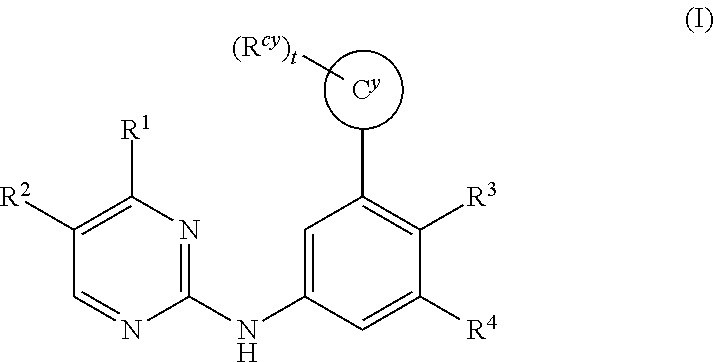
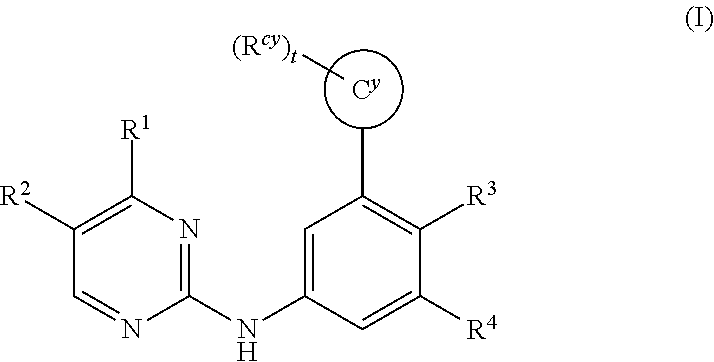
Substituted diazine and triazine spleen tyrosine kinease (SYK) inhibitors
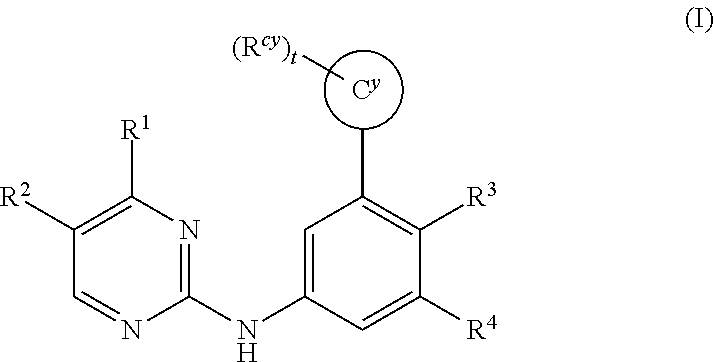
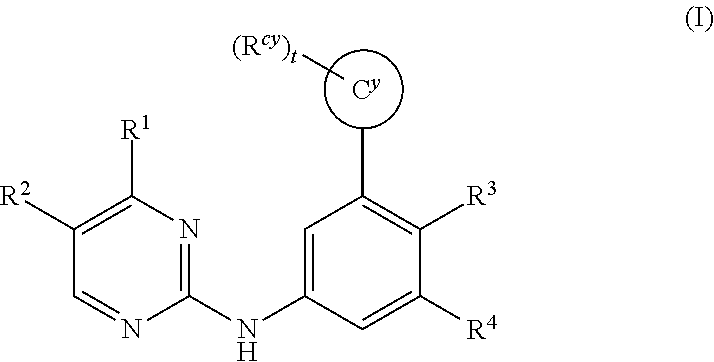
The invention provides certain substituted diazine and triazine compounds of the Formula (I) (I) or pharmaceutically acceptable salts thereof, wherein R1, R2, R3, R4, Rcy, Cy, and t are as defined herein. The invention also provides pharmaceutical compositions comprising such compounds, and methods of using the compounds for treating diseases or conditions mediated by Spleen Tyrosine Kinase (Syk) kinase.
Owner:MERCK SHARP & DOHME LLC
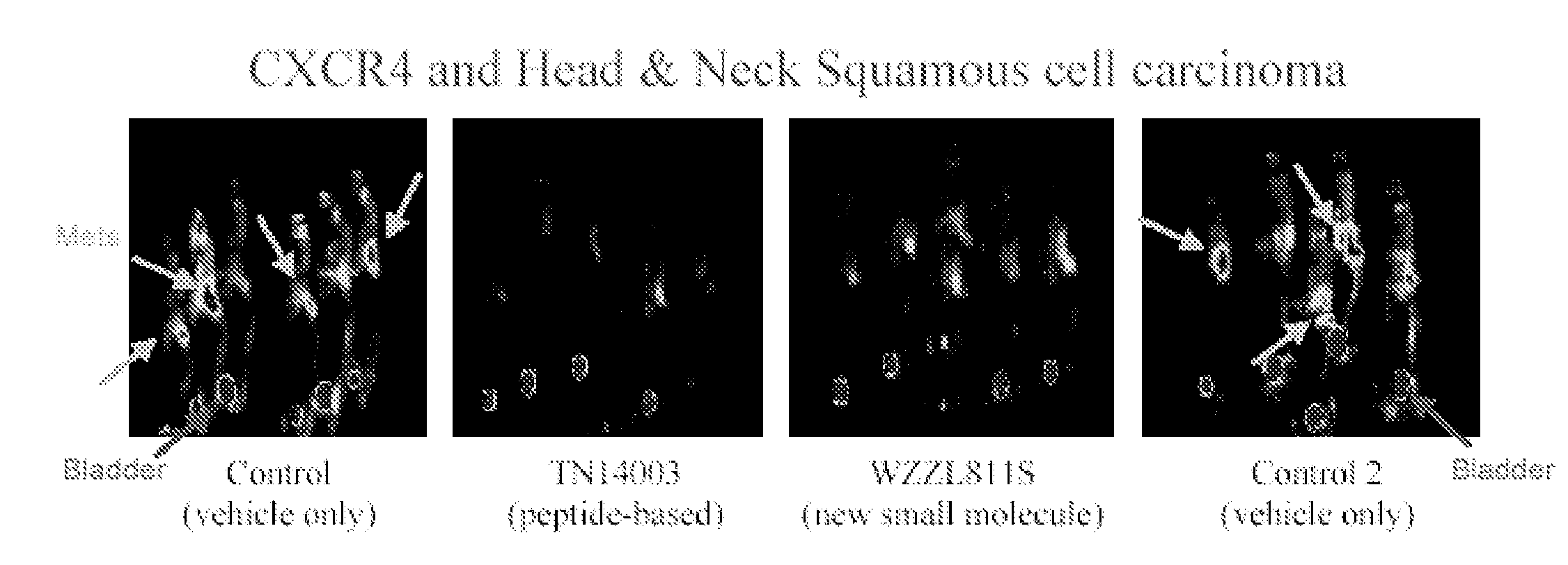
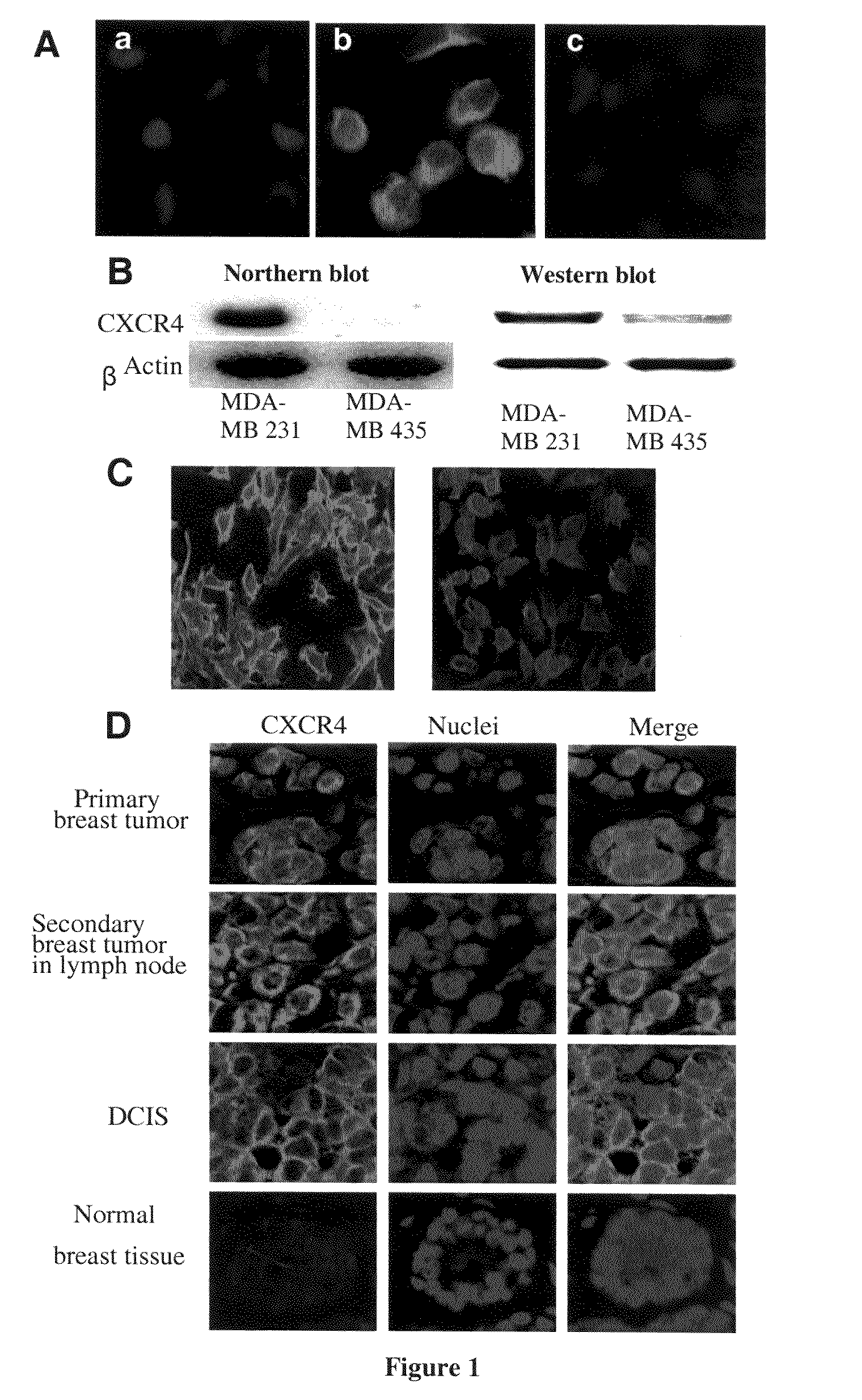
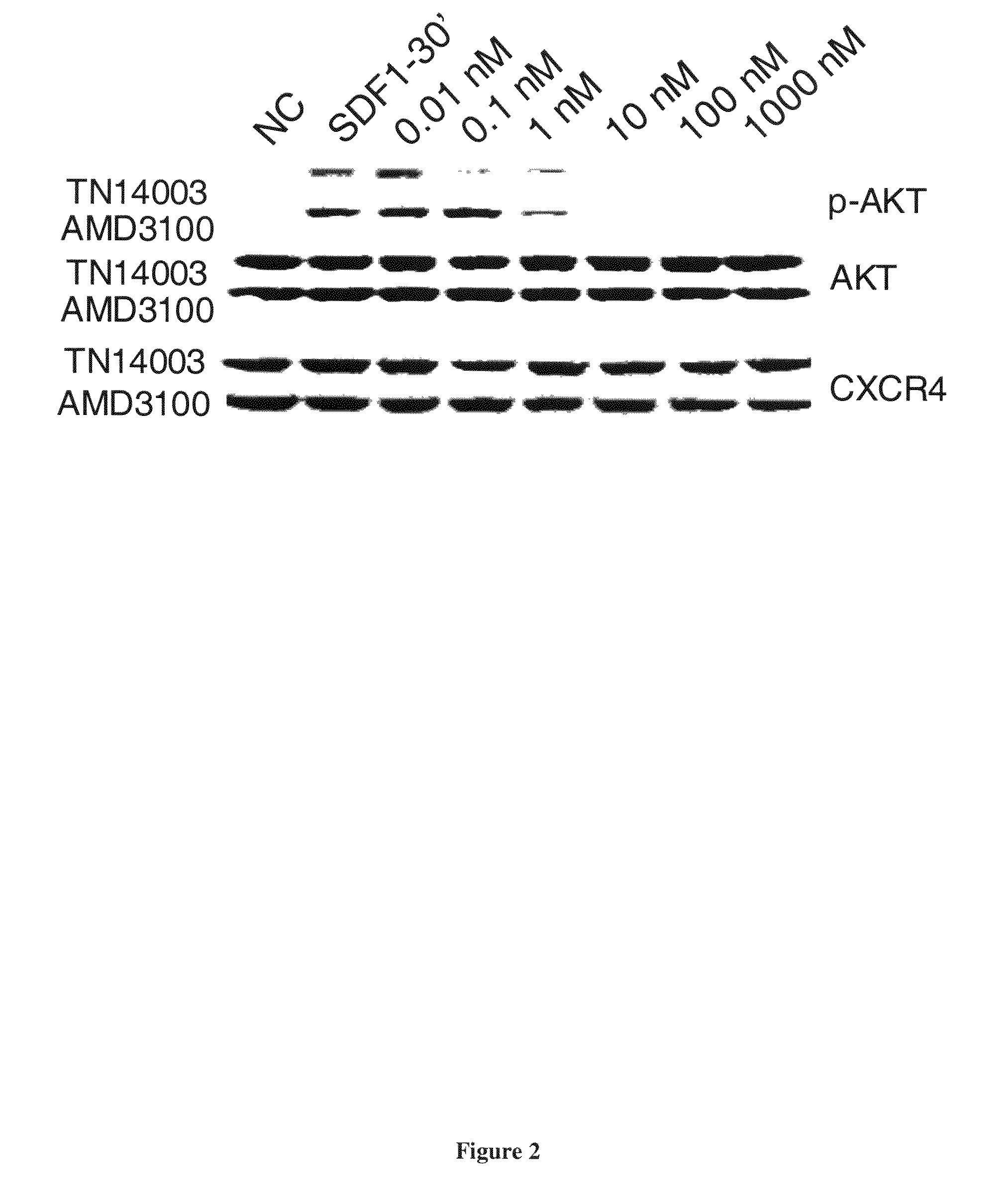
CXCR4 Antagonists Including Diazine And Triazine Structures For The Treatment Of Medical Disorders
ActiveUS20090099194A1Inhibit transferReduce the possibilityBiocideOrganic active ingredientsDiseaseMedical disorder
The invention provides compounds, pharmaceutical compositions and methods of use of certain compounds that are antagonists of the chemokine CXCR4 receptor for the treatment of proliferative conditions mediated by CXCR4 receptors or for the treatment of viral infections. The compounds provided interfere with the binding of SDF 1 to the receptor. These compounds are particularly useful for treating or reducing the severity of hyperproliferative diseases by inhibiting metastasis, or for reducing entry of HIV in to a cell while not reducing the capacity of the stem cells to proliferate. The compounds may be useful for long term treatment regimes.
Owner:EMORY UNIVERSITY
Anti-corrosion agents for transparent conductive film
InactiveCN102939346AAzine dyesNon-conductive material with dispersed conductive materialDiazineTransparent conducting film
Owner:CARESTREAM HEALTH INC
Anti-corrosion agents for transparent conductive film
InactiveUS20130004765A1Material nanotechnologySynthetic resin layered productsTransparent conducting filmDiazine
Owner:CARESTREAM HEALTH INC
Pigment grade corrosion inhibitor host-guest compositions and procedure
InactiveUS20050022693A1High chemistryEffective corrosionPigmenting treatmentOther chemical processesO-Phosphoric AcidPyrrole
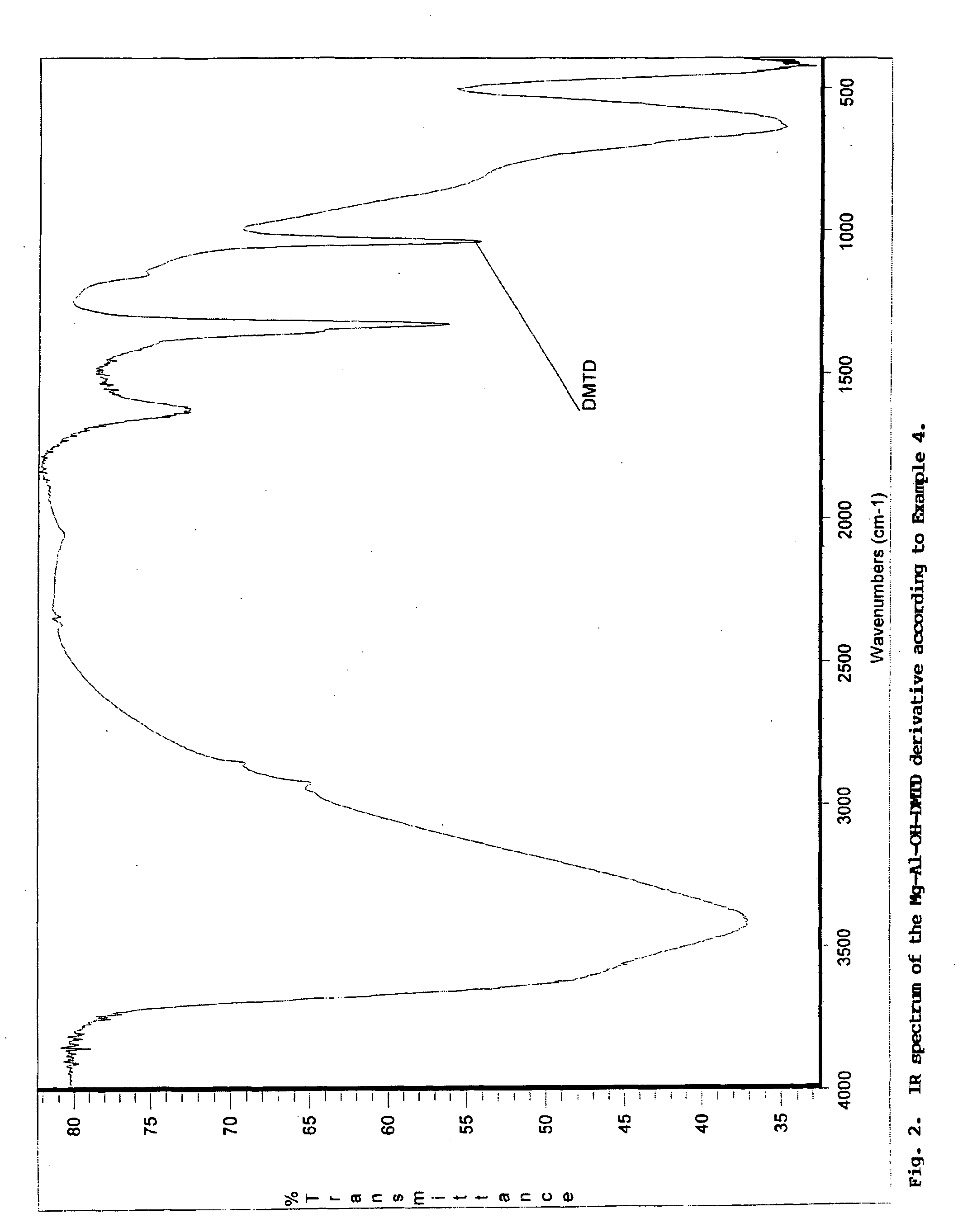
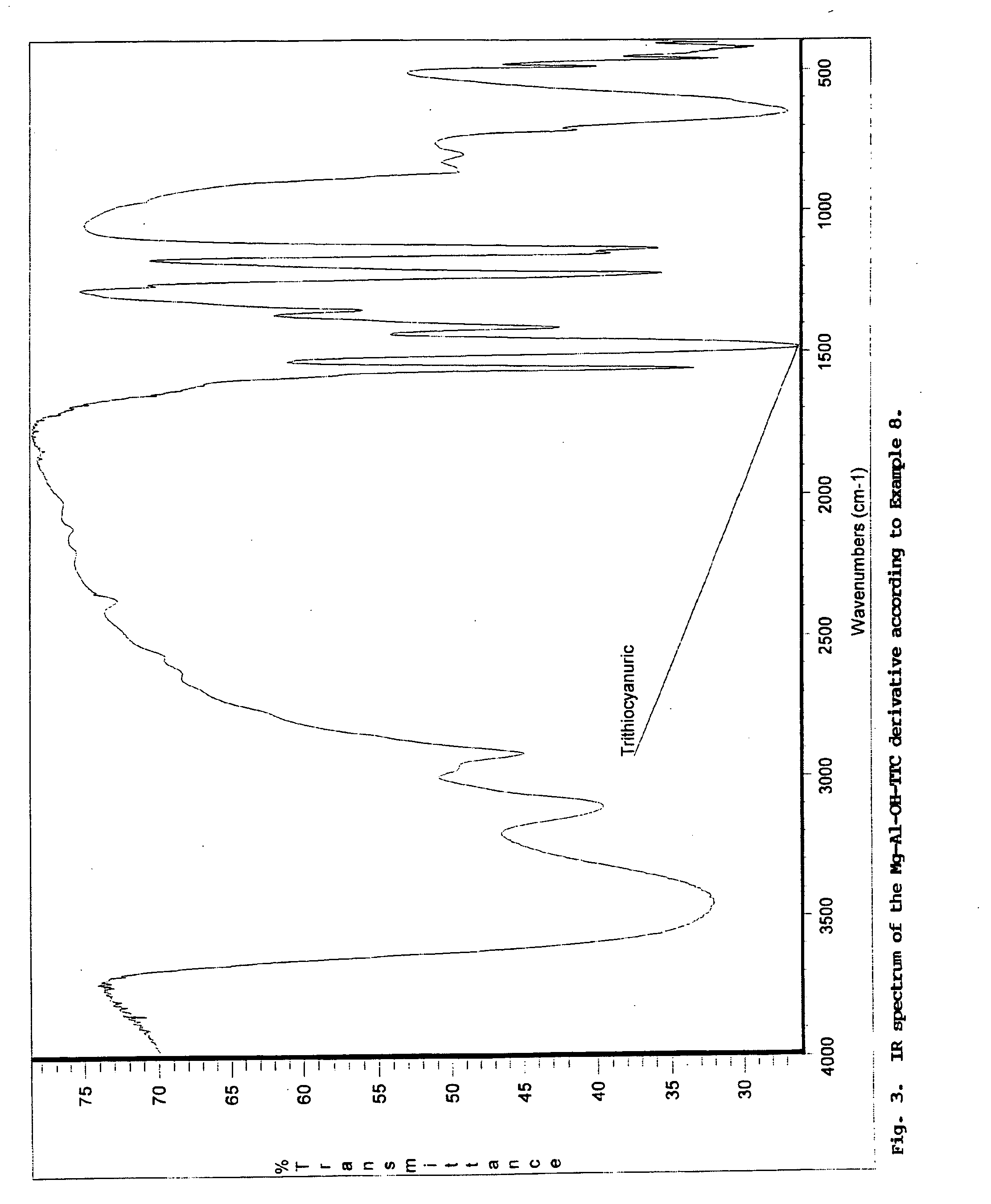
A pigment grade corrosion inhibitor and a method of forming the inhibitor is disclosed. The inhibitor is comprised of a host species comprised of an inorganic compound having a layered structure and a guest species comprised an anionic species of a weak acid. The host species is preferably a double hydroxide having a structure of: [M(II)1-xM(III)x(OH−)2] [An−x / n.y H2O], where M(II) is a divalent metal cationic species, M(III) is a trivalent metal cationic species, and An− is an anionic species, preferably with the species present in a range of: 0.2≦M(III) / (M(II)+M(III))≦0.4. The guest species include: ortho-phosphoric, pyrophosphoric, tripoly-phosphoric, polyphosphoric acid; mono- and di-alkyl or aryl-esters of ortho-phosphoric and pyrophosphoric acid; metaphosphoric, trimeta-phosphoric, poly-metaphosphoric acid; phosphorous (phosphonic) acid; derivatives of phosphonic acid; alkyl and aryl esters of thio-phosphoric and dithio-phosphoric acid; molybdic, phospho-molybdic, silico-molybdic acid; boric acid; cyanamidic acid; nitrous acid; derivatives of thio- and dithiocarbonic acid, including o-alkyl esters; derivatives of dithiocarbamic acid, including N-alkyl dithiocarbamates; pyrrolidinecarbodithioic acid; thio-organic compounds functionalized with at least one —SH group of acidic character, including: 2,5-dimercapto-1,3,4-thiadiazole (DMTD), 2,4-dimercapto-s-triazolo-[4,3-b]-1,3-4 thiadiazole, trithiocyanuric acid (TMT), and dithiocyanuric acid, various N—,S— and N,N—, S,S— and N,S-substituted derivatives of the above DMTD and TMT compounds; various S-substituted derivatives of trithiocyanuric acid; dimer and polymer derivatives of the above DMTD and TMT compounds, including 5,5′ dithio-bis (1,3,4 thiadiazole-2(3H)-thione or (DMTD)2, and (DMTD)n, polymers of DMTD and (TMT)2, dimers and polymers of TMT; various combinations of all the above; soluble salts of DMTD and TMT; poly-ammonium salt of DMTD or (DMTD)n and TMT formed with polyamines; mercapto-benzothiazole, mercapto-benzoxazole, mercapto-benzimidazole, and combinations, thereof; di- and poly-mercapto compounds, including: di-mercapto derivatives of thiophene, pyrrole, furane, diazoles, and thiadiazoles; di- and tri-mercapto derivatives of pyridine, diazines, triazines, benzimidazole, and benzothiazole, including dimercaptopyridine, 2, 4-dithiohydantoine, and 2,4,-dimercapto-6-amino-5-triazine; and carboxylic and di-carboxylic acids, including ascorbic, salicylic acid, phthalic acid, nitro-phthalic acid, succinic acid, and derivatives of succinic acid, including 1-(benzothiazol-2-ylthio)succinic acid.
Owner:LUMIMOVE
Chemical methods for producing tagged nucleotides
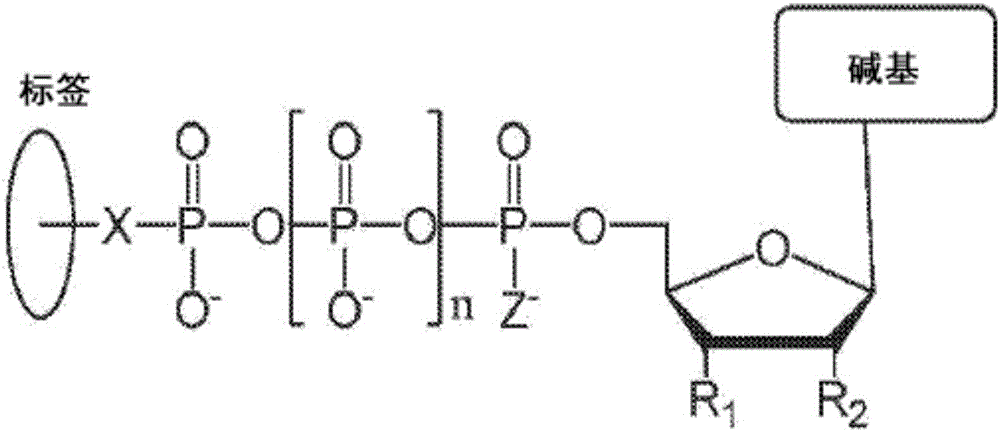
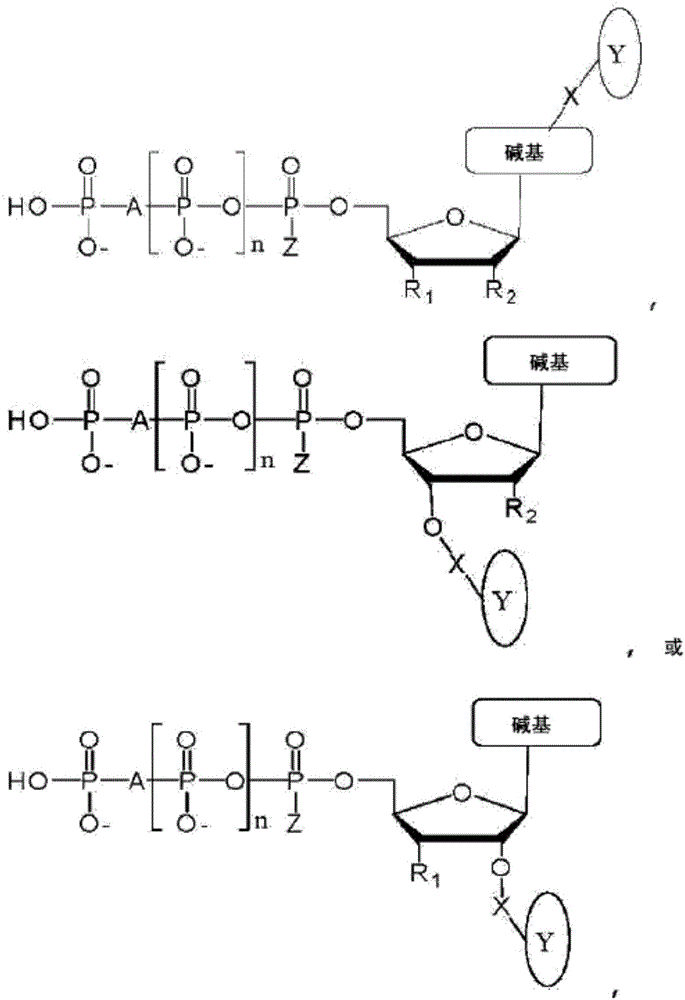
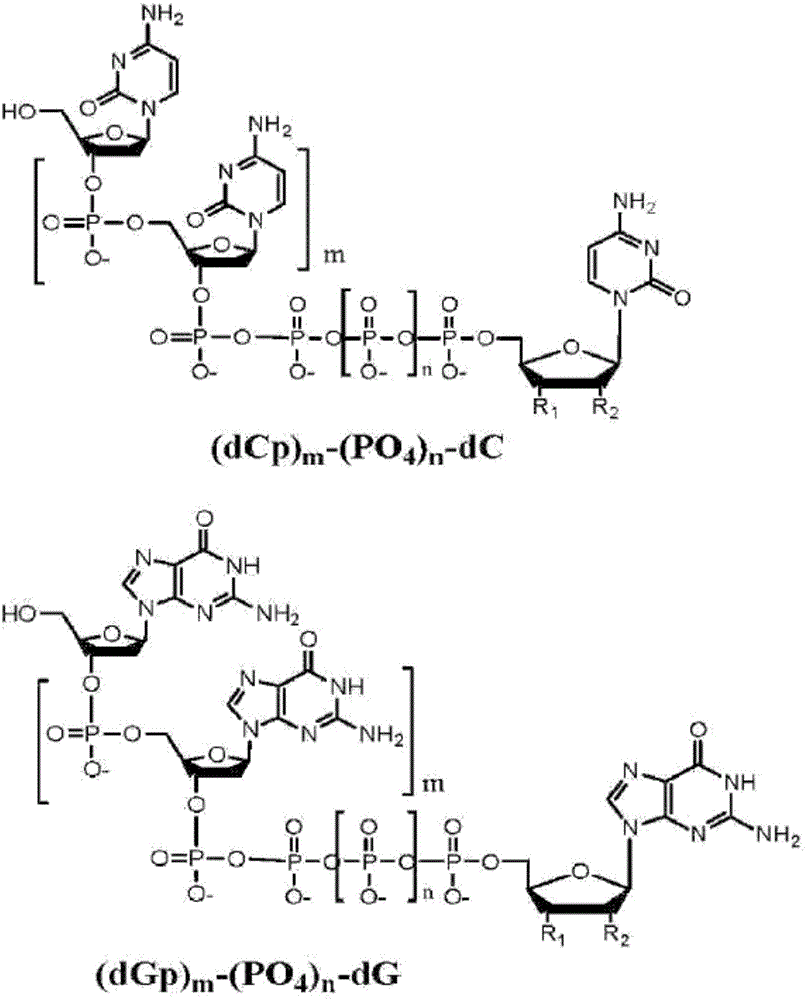
This disclosure provides systems and methods for attaching nano pore-detectable tags to nucleotides. The disclosure also provides methods for sequencing nucleic acids using the disdosed tagged nudeotides. Provided herein are nudeotides with attached tags and methods for attaching tags to nudeotides. The tags can be attached by chemical reactions, such as 'dick chemistry'. In an aspect, the present disdosure provides a tagged nudeotide, comprising: (a) a poly-phosphate moiety having a terminal phosphate; and (b) a tag covalently coupled to the terminal phosphate of the nudeotide by a triazole, a 1,2-diazine, a disulfide, a secondary amine, a hydrazone, a thio-acetamide, or a maleimide-thioadduct.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Space-through charge transfer compound, and organic light emitting diode and display device using the same
ActiveUS20160111650A1Improve emission efficiencyEmission efficiency is highOrganic chemistrySolid-state devicesCarbazoleElectron donor
Discussed is a space-through charge transfer compound including a naphthalene core; an electron donor moiety selected from carbazole and phenylcarbazole; and an electron acceptor moiety selected from pyridine, diazine, triazole, and phenyl benzodiazole, wherein the electron donor moiety and the electron acceptor moiety are combined to first and eighth positions of the naphthalene core with a benzene linker, respectively.
Owner:LG DISPLAY CO LTD
Process for preparing polyisocyanates containing iminooxadiazinedione groups
The present invention relates to process for preparing a polyisocyanate containing an iminooxadiazinedione group by trimerizing a portion of the isocyanate groups of a polyisocyanate, which does not contain iminooxadiazinedione groups, in the presence of a catalyst containing an anion corresponding to formula I)(Rf)n—CR′(3-n)—C(O)O− (I)whereinRf is a perfluorinated C1-C30 radical which is optionally branched, cyclic and / or unsaturated,R′ are identical or different, optionally heteroatom-containing substituents selected from H, C1-C20 alkyl and / or aryl andn is 1 or 2.
Owner:COVESTRO DEUTSCHLAND AG
Substituted 1,2,3-Triylidenetris(cyanomethanylylidene) Cyclopropanes for VTE, Electronic Devices and Semiconducting Materials Using Them
ActiveUS20170373251A1High yieldHigh purityOrganic chemistrySolid-state devicesTetrafluoroethyleneDopant
The present invention relates to a process for preparation of an electrically doped semiconducting material comprising a [3]-radialene p-dopant or for preparation of an electronic device containing a layer comprising a [3]-radialene p-dopant, the process comprising the steps: (i) loading an evaporation source with the [3]-radialene p-dopant; and (ii) evaporating the [3]-radialene p-dopant at an elevated temperature and at a reduced pressure, wherein the [3]-radialene p-dopant is selected from compounds having a structure according to formula (I) wherein A1 and A2 are independently aryl- or heteroaryl-substituted cyanomethylidene groups, the aryl and / or heteroaryl is selected independently in A1 and A2 from 4-cyano-2,3,5,6-tetrafluorphenyl,2,3,5,6-tetrafluorpyridine-4-yl, 4-trifluormethyl-2,3,5,6-tetrafluorphenyl, 2,4-bis(trifluormethyl)-3,5,6-trifluorphenyl, 2,5-bis(trifluormethyl)-3,4,6-trifluorphenyl, 2,4,6-tris(trifluormethyl)-1,3-diazine-5-yl, 3,4-dicyano-2,5,6-trifluorphenyl, 2-cyano-3,5,6-trifluorpyridine-4-yl, 2-trifluormethyl-3,5,6-trifluorpyridine-4-yl, 2,5,6-trifluor-1,3-diazine-4-yl and 3-trifluormethyl-4-cyano-2,5,6-trifluophenyl), and at least one aryl or heteroaryl is 2,3,5,6-tetrafluorpirydine-4-yl, 2,4-bis(trifluormethyl)-3,5,6-trifluorphenyl, 2,5-bis(trifluormethyl)-3,4,6-trifluorphenyl, 2,4,6-tris(trifluormethyl)-1,3-diazine-5-yl, 3,4-dicyano-2,5,6-trifluorphenyl, 2-cyano-3,5,6-trifluorpyridine-4-yl, 2-trifluormethyl-3,5,6-trifluorphenyl, provided that the heteroaryl in both A1 and A2 cannot be 2,3,5,6-tetrafluorpyridine-4-yl at the same time, respective [3]-radialene compounds, and semiconducting materials and layer, and electronic devices comprising said compounds.
Owner:NOVALED GMBH
Anticorrosion agents for transparent conductive film
InactiveUS20140072826A1Improve efficiencyNon-conductive material with dispersed conductive materialCoatingsPreservativeTransparent conducting film
Owner:CARESTREAM HEALTH INC
Substituted diazine and triazine spleen tyrosine kinease (Syk) inhibitors
The invention provides certain substituted diazine and triazine compounds of the Formula (I) (I) or pharmaceutically acceptable salts thereof, wherein R1, R2, R3, R4, Rcy, Cy, and t are as defined herein. The invention also provides pharmaceutical compositions comprising such compounds, and methods of using the compounds for treating diseases or conditions mediated by Spleen Tyrosine Kinase (Syk) kinase.
Owner:MERCK SHARP & DOHME LLC
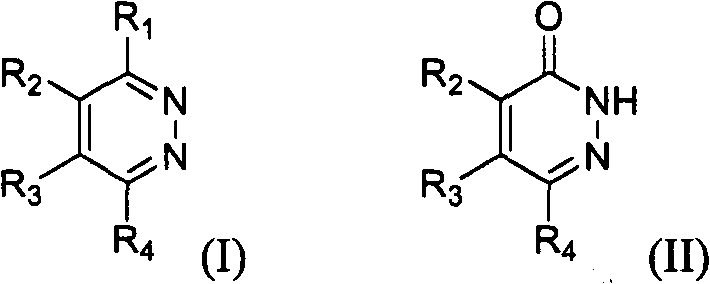
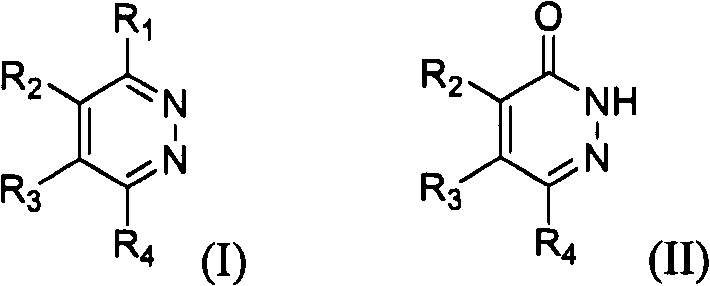
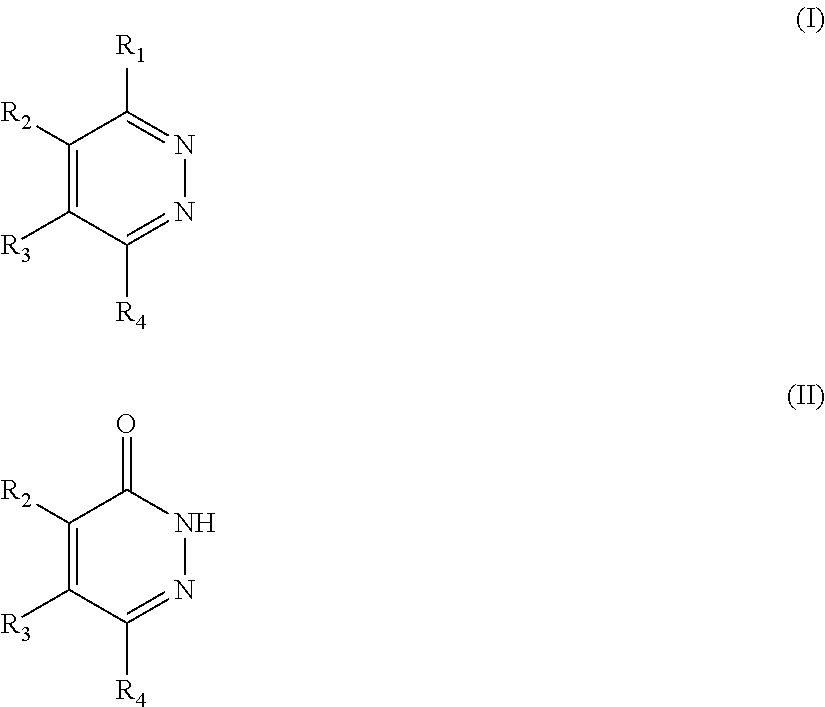
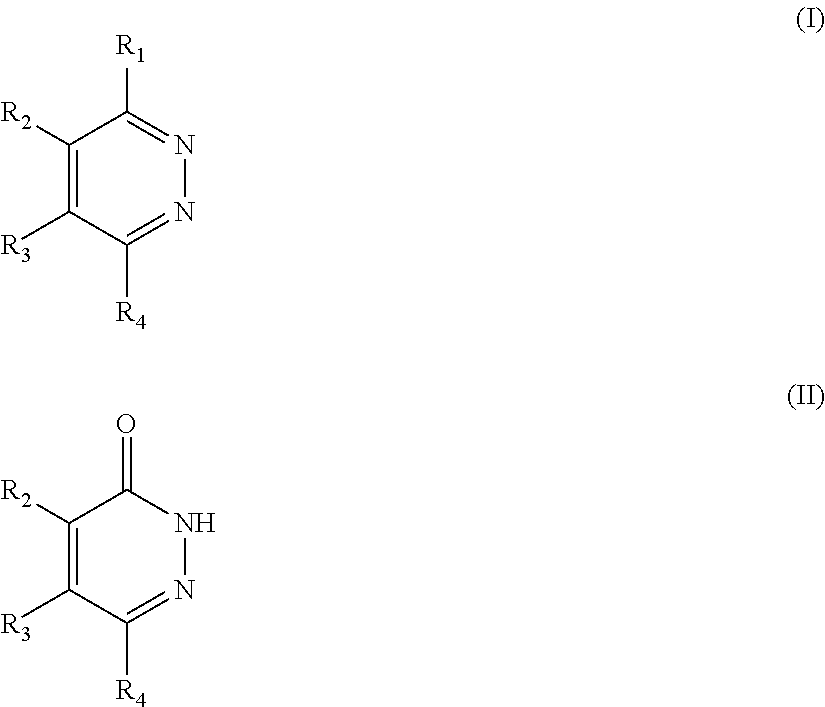
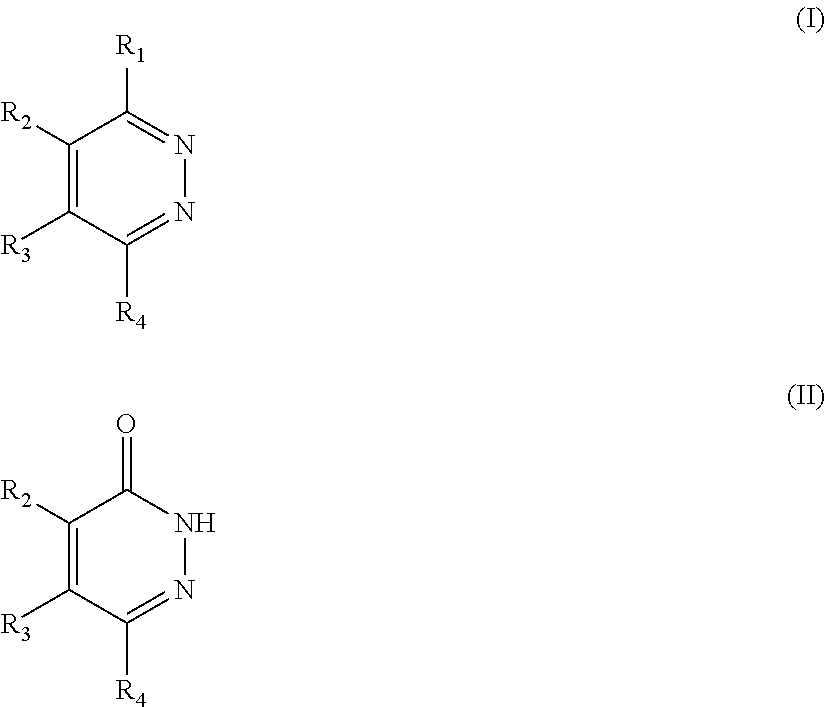
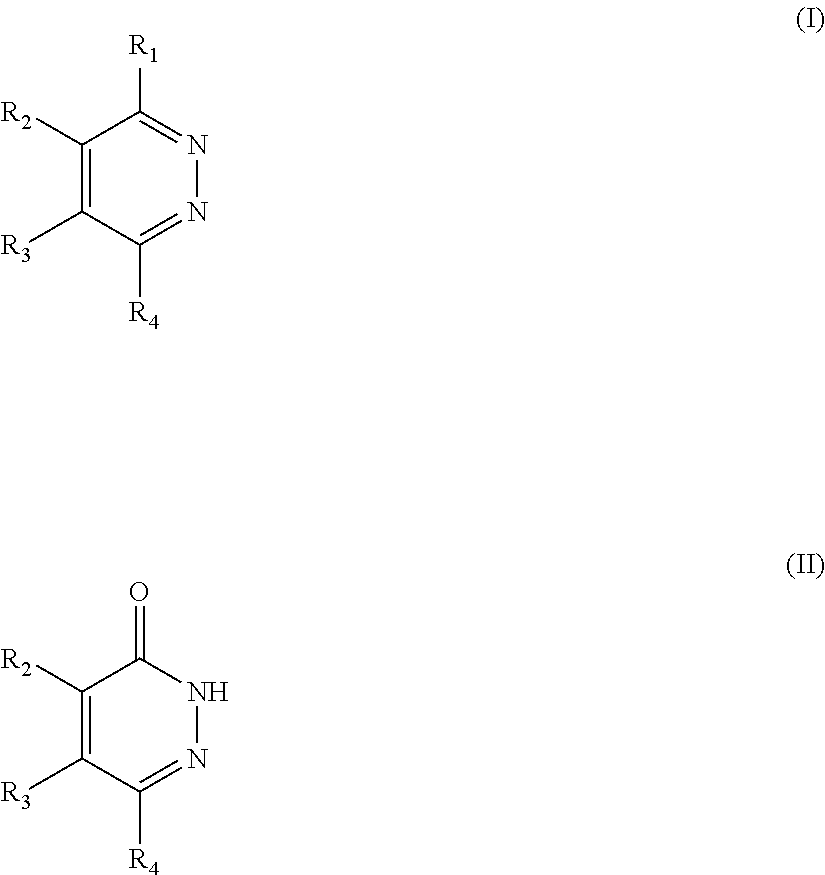
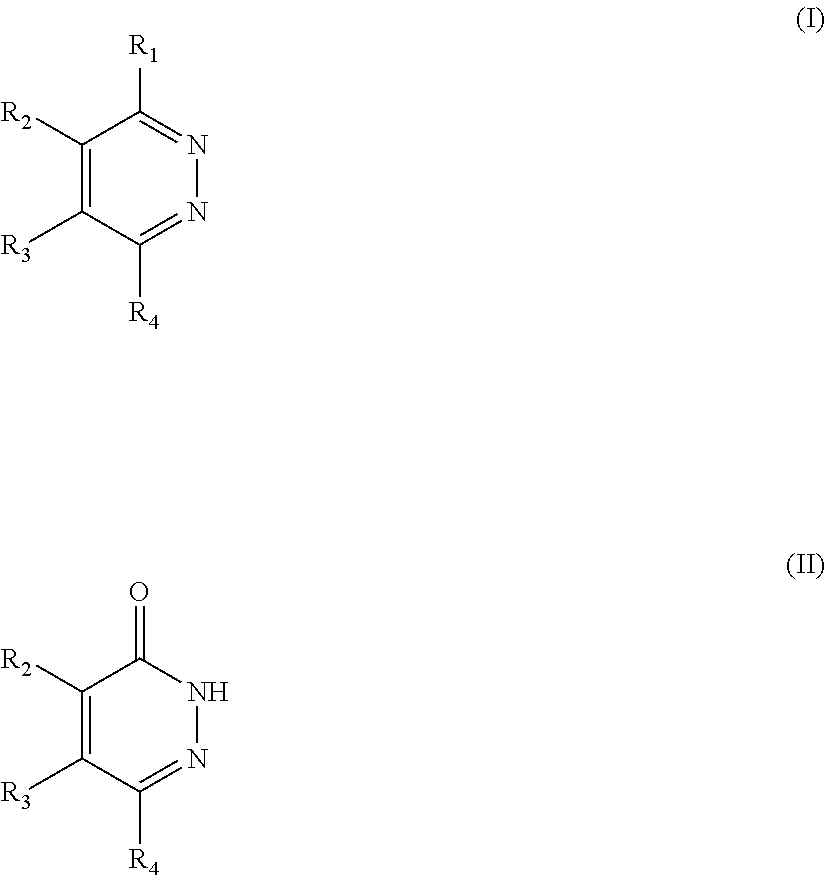
Novel diazine derivatives
The present invention provides compounds of formula (I): their pharmaceutically acceptable salts or esters, enantiomeric forms, diastereoisomers and racemates, the preparation of the above-mentioned compounds, pharmaceutical compositions containing them and their manufacture, as well as the use of the above-mentioned compounds in the control or prevention of illnesses such as cancer.
Owner:F HOFFMANN LA ROCHE INC
Space-through charge transfer compound, and organic light emitting diode and display device using the same
ActiveUS10141515B2Emission efficiency is highImprove emission efficiencyOrganic chemistrySolid-state devicesCarbazoleElectron donor
Owner:LG DISPLAY CO LTD
Electrolytic solution for secondary battery and secondary battery containing the same
ActiveUS7312000B2Improve securityImprove battery safetyOrganic electrolyte cellsSolid electrolyte cellsHydrogen atomPhotochemistry
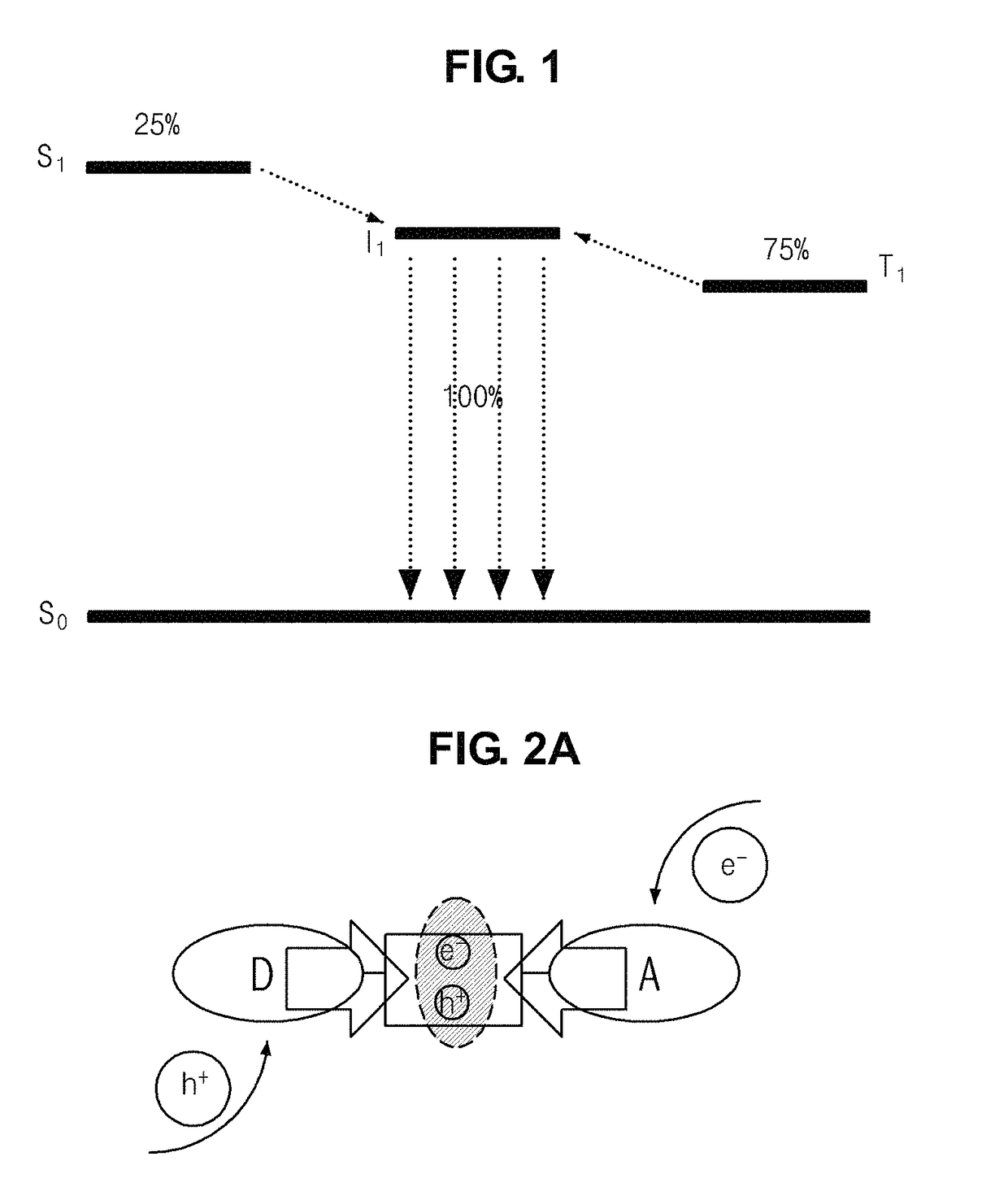
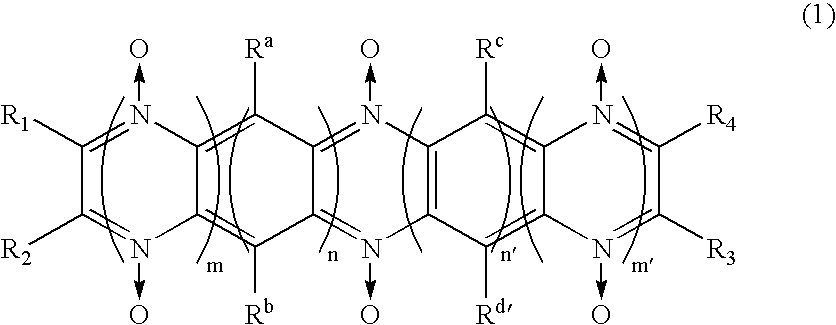
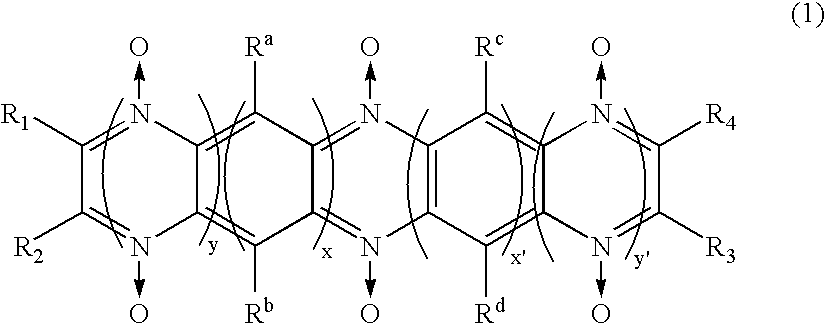
A battery, which has excellent safety in an overcharged state and is excellent in low-temperature characteristics and cycle characteristics, is provided.The electrolytic solution of the secondary battery contains a compound which has a diazine N,N′-dioxide structure represented by general formula (1).in which, n, m, n′, and m′ each independently are an integer 0 or larger; the diazine rings and the benzene rings may be condensed alternately or randomly; and substituents R1, R2, R3, R4, Ra, Rb, Rc, and Rd each independently represent hydrogen atom, halogen atom, or specific group. However, when n is 2 or larger, then Ra and Rb may be the same or different, and when n′ is 2 or larger, then Rc and Rd may be the same or different. In addition, these substituents, in cooperation with each other, may form a ring structure.
Owner:NEC CORP
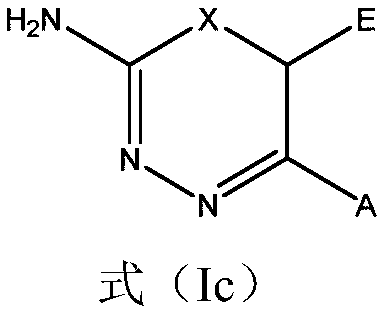
2-amino-1,3,4-thiadiazine and 2-amino-1,3,4-oxadiazine based antifungal agents
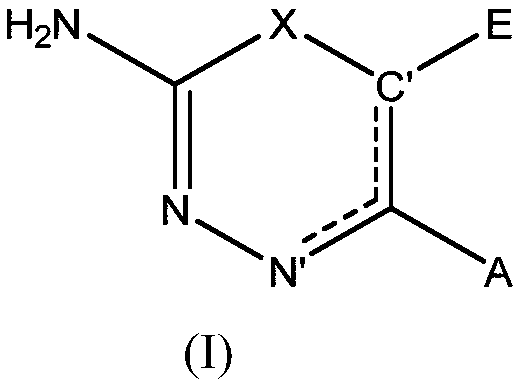
The invention provides a compound which is a diazine of formula (I) or a tautomer thereof, or a pharmaceutically acceptable salt thereof, for use as an antifungal agent. The formula (I) is shown in the specification, wherein X, N', C', A and E are as defined herein. The invention also provides the compound of Formula (I) as defined herein.
Owner:F2G LTD
Intermediate for preparing rosuvastatin and preparation method and application thereof
ActiveCN102584717AReduce usageLow priceGroup 4/14 element organic compoundsGroup 5/15 element organic compoundsHydrogenHalogen
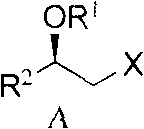
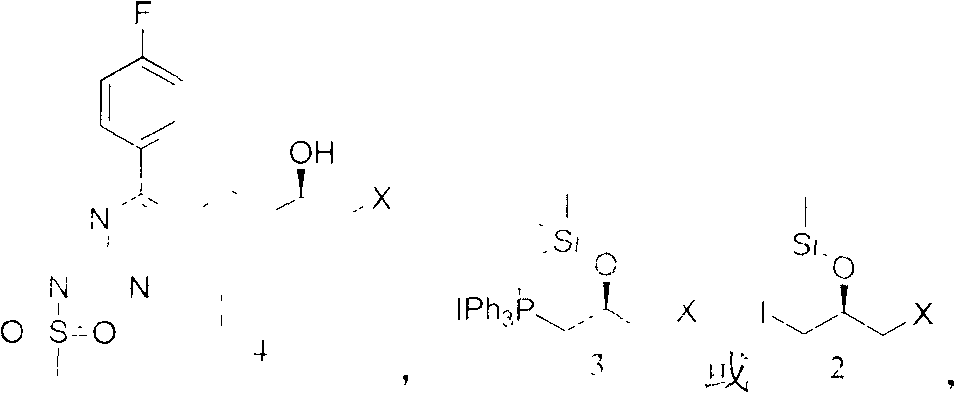
The invention relates to the technical field of heterocyclic chemistry, particularly relates to the technical field of the heterocyclic chemistry containing 1,3-diazine cyclic, specifically relates to an intermediate for preparing rosuvastatin and a preparation method and application. An intermediate compound for preparing the rosuvastatin has the following structure as shown in the formula (A), wherein X is halogen, R1 is hydrogen or trimethyl silane, R2 is iodine methyl or a group with the structure in the following formula.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Nickel-catalyzed heterocyclic phosphonium salt and aryl bromide direct reduction cross-coupling method and product
ActiveCN113387876AExpand the range of prepared substratesSimple post-processingOrganic substitutionArylPhosphonium salt
The invention discloses a nickel-catalyzed heterocyclic phosphonium salt and aryl bromide direct reduction cross-coupling method and a product, and the method comprises the following steps: in a nitrogen atmosphere, heating a mixture of magnesium chips and lithium chloride; cooling the mixture to room temperature, and adding an ultra-dry solvent into the mixture; then respectively adding a phosphonium salt compound, a catalyst, a ligand and aryl bromide, and stirring to react; and quenching, washing, extracting and drying the reaction product, and separating by column chromatography to obtain the arylated pyridine or diazine compound. The preparation method has the characteristics of mild reaction conditions, simple post-treatment, green steps, low pollution, high economic benefits and the like.
Owner:NANJING UNIV OF TECH
Mould-proof metal working fluid
InactiveCN104479830AImprove rust resistanceImprove the lubrication effectLubricant compositionMetal working fluidPolyethylene glycol
The invention discloses a mould-proof metal working fluid which is formed by mixing materials in parts by weight as follows: 46-55 parts of castor oil, 33-45 parts of rapeseed oil, 22-26 parts of petroleum sodium sulfonate, 11-21 parts of polyethylene glycol, 12-15 parts of sodium dodecyl benzene sulfonate, 8-10 parts of sodium molybdate, 3-5 parts of petroleum sodium sulfonate, 13-15 parts of dimethyl polysiloxane, 3-10 parts ofemulsified silicone oil and 2-8 parts ofdihydro-diazine. The mould-proof metal working fluid has the advantages of good rust-proof effect, excellent lubricationproperty, capability of effectively preventing mold from being generated on casting surface as well as better mould-proof effect.
Owner:CHANGSHU JIANGNAN FORGING
Formulations for killing agricultural pests
ActiveUS20190075793A1Reduce resistanceLower Level RequirementsBiocideDead animal preservationCarbamateDouble bond
Killing one or more agricultural pests is accomplished by bringing the agricultural pests into contact with a toxic amount of a formulation comprising: (I) one or more compounds selected from the group consisting of: (1) one or more compounds of structure (A), wherein the compounds of structure (A) comprise:wherein R is selected from the group consisting of —OH, ═O, —OC(O)R4, —OR6, —(OR6)2, wherein each R6 is independently selected from an alkyl group containing from 1 to 4 carbon atoms and R is a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to two double bonds and from 1 to 15 carbon atoms; X is O or CH2, with the proviso that when X is O, then R can only be ═O; each Z is independently selected from the group consisting of (CH) and (CH2); y is a numeral selected from 1 and 2; R1 is selected from the group consisting of H or a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to two double bonds and from 1 to 15 carbon atoms; R2 is selected from the group consisting of H and a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to three double bonds and from 1 to 15 carbon atoms; R3 is selected from the group consisting of H and a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to three double bonds and from 1 to 15 carbon atoms, —(CH2)nOH, —C(O)OR5, —CH2C(O)OR7, —CH2C(O)R8, —C(O)NR9R10, —CH2C(O)NR11R12 where each of R5, R7, R8, R9, R10, R11 and R12 is independently selected from H and a branched or straight chain, saturated or unsaturated hydrocarbyl group with zero to three double bonds and from 1 to 15 carbon atoms and n is an integer of from 1 to 12; the bond between the 2 and 3 positions in the ring structure may be a single or a double bond; and wherein the compounds of structure (A) contain from 11 to 20 total carbon atoms; and (2) one or more sesquiterpenes selected from the group consisting of acyclic, monocyclic, bicyclic and tricyclic sesquiterpenes and derivatives thereof; and (II) one or more toxicant compounds selected from the group consisting of carbamates, organochlorines, pyrethroids, sulfoximines, neonicotinoids, pyridine azomethines, diamides, organophosphates, phenylpyrazoles, oxadiazines, and ketoenols. The formulation can be a synergistic formulation.
Owner:BEDOUKIAN RES
Method for degrading organophosphorus pesticide
ActiveCN111675306AImprove degradation efficiencyPromote degradationWater contaminantsEnergy based wastewater treatmentPotassium persulfateDiazinon
The invention relates to the field of degradation of pesticide-containing wastewater, in particular to a method for degrading organophosphorus pesticide. The method comprises putting an organophosphorus pesticide solution into a closed reaction water tank, adding an inorganic oxidant, and stirring to degrade the organophosphorus pesticide, wherein the organophosphorus pesticide is diazinon, the inorganic oxidant is one or more than two of potassium persulfate, sodium perchlorate and potassium periodate and the molar ratio of the inorganic oxidant to the organophosphorus pesticide is 2: 1. Themethod is low in treatment cost, simple to operate and free of secondary pollution, and the degradation rate of diazinon in the wastewater after the inorganic oxidant is added can reach 80% or above.
Owner:LIAONING UNIVERSITY
Diazinon coated slow-released granule preparation method
InactiveCN106900702AReduce releaseReduce the amount of applicationBiocideAnimal repellantsChemistryDiazinon
The invention discloses a diazinon coated slow-released granule preparation method which mainly includes the steps: preparing suspension liquid containing diazinon; preparing diatomite granules loading pesticides; preparing coating agents and coatings. According to the method, the suspension liquid of the diazinon is injected into holes of the diatomite granules, diazinon molecules adhere in the holes of the diatomite granules through Van der Waals force, explosion of the pesticides is avoided, the outsides of the diatomite granules loading the pesticides are coated, and release of the diazinon is further slowed. Compared with original pesticides, diazinon coated slow-released granules prepared by the method have the advantages of slow release and long effect, application amount is greatly reduced, use ratio is greatly improved, effects of the original pesticides preventing diseases and insect pests can be improved, agricultural production cost is reduced, environmental pollution caused by the pesticides can be reduced or avoided, and the diazinon coated slow-released granule has good environmental protection effect.
Owner:浙江皓翔矿业有限公司
Prepolymers based on di- or polyisocyanates and formamide-terminated low molecular weight compounds, processes for preparing the same and uses thereof
InactiveUS20110015291A1Low viscosityUrea derivatives preparationIsocyanic acid derivatives preparationAllophaneOrganic group
Prepolymers, which are accessible from formamides of low molecular weight di- or triamines (formamide-terminated low molecular weight compounds) and di- or polyisocyanates, of the general formula (II):X—[—N(CHO)—CO—NH—R1—NCO]n (II)wherein X represents a linear or branched aliphatic, cycloaliphatic, heterocyclic and / or aromatic structural unit having 2 to 40 carbon atoms, and which is optionally further substituted and / or optionally comprises one or more heteroatoms, wherein R1 represents an organic radical which may optionally contain one or more heteroatoms and which may further contain one or more additional free isocyanate groups and / or one or more urethane, biuret, carbodiimide, isocyanurate, allophanate, iminooxadiazinedione and / or uretdione structural units, and wherein n≧2; processes for making the same; compositions containing the same; and uses thereof
Owner:COVESTRO DEUTSCHLAND AG
Electrode-active material and electrodes made by using the same, and cells equippe with the electrodes
InactiveUS20050100787A1High capacity densityStable functionOrganic electrolyte cellsActive material electrodesBenzeneOligomer
An object of the present invention is to provide a battery which is high in capacity density and superior in stability. An electrode containing a compound having a diazine-N,N′-dioxide structure shown by a general formula (1) described below as an electrode active material is used, where x, y, x′, and y′ independently shows integer numbers of 0 or more respectively, and the order of condensation of diazine rings and benzene rings may be alternate or random. One of substituents R1, R2, R3, R4, Ra, Rb, Rc, and Rd shows a part of main chain or side chain of oligomer or polymer, and the other independently shows hydrogen atom, halogen atom, or a specific group.
Owner:NEC CORP
Material for light-emitting apparatus and electron transport layer, organic compound, light-emitting apparatus, light-emitting device, electronic equipment, and lighting device
PendingCN113563269ALow refractive indexCarrier TransportOrganic chemistrySolid-state devicesSimple Organic CompoundsRefractive index
Provided is a material for a light emitting apparatus having a low refractive index. The material for the light emitting apparatus contains an organic compound having a pyridine skeleton, a diazine skeleton, or a triazine skeleton in which the ratio of carbon atoms bonded to the sp3 hybrid orbital is within a prescribed range. The material for the light emitting apparatus includes an organic compound in which a heteroaromatic ring including at least one six-membered ring including one to three nitrogen atoms, a plurality of aromatic hydrocarbon rings having 6 to 14 ring-forming carbon atoms, at least two of the plurality of aromatic hydrocarbon rings being benzene rings, and a plurality of hydrocarbon groups bonded in an sp3 hybrid orbital are included. The layer comprising the organic compound has an ordinary light refractive index of 1.5 or more and 1.75 or less with respect to light having an arbitrary wavelength in the range of 455 nm or more and 465 nm or less.
Owner:SEMICON ENERGY LAB CO LTD
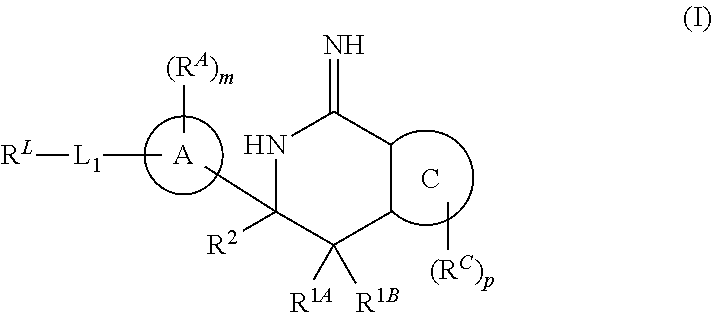
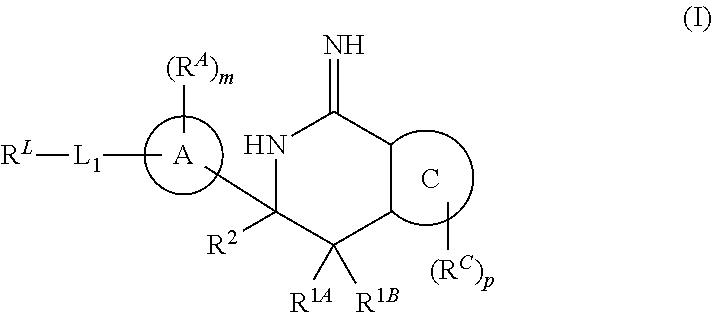
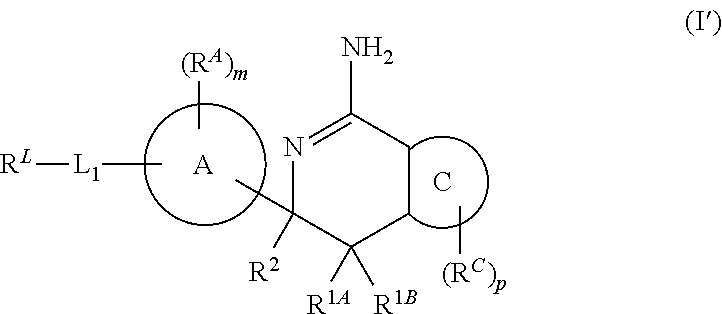
Diazine-fused amidines as BACE inhibitors, compositions, and their use
In its many embodiments, the present invention provides certain C-6 spirocarbocyclic iminothiadiazine compounds, including compounds Formula (I) or a tautomer thereof, and pharmaceutically acceptable salts of said compounds and said tautomers, wherein R1A, R1B, R2, RA, ring A, RA, m, L1, RL, ring C, RC, and p are as defined herein. The novel compounds of the invention are useful as BACE inhibitors and / or for the treatment and prevention of various pathologies related thereto. Pharmaceutical compositions comprising one or more such compounds (alone and in combination with one or more other active agents), and methods for their preparation and use, including for the possible treatment of Alzheimer's disease, are also disclosed.
Owner:MERCK SHARP & DOHME LLC
Method for preparing carbazole diazine organic pigment
The invention discloses a method for preparing a carbazole diazine organic pigment. The method comprises the following steps: adding a crude product of a pigment, namely purple P. V. 23, into grindingequipment, and performing crushing grinding at a certain temperature; putting the grinded crude product of the pigment into a kneading machine, adding an inorganic salt grinding aid and an adhesive into the kneading machine, and performing grinding for a certain time at a certain temperature so as to obtain a pigment mixture; adding water, performing pulping, performing water washing to remove the adhesive and the inorganic salt grinding aid, and performing drying and crushing so as to obtain dark purple powder; adding a pigment dispersant into the prepared dark purple powder, and performinguniform mixing, so as to obtain a final desired product. The pigment, namely purple P. V. 23, which is prepared by using the method, has good pigment coloring performance and a red phase, and meanwhile is low in ink system viscosity, good in flowability and high in stability when being applied to a solvent type ink system.
Owner:河北捷虹颜料化工有限公司
Compound and composition for forming organic film
ActiveUS11022882B2Superior gap filling and planarizing characteristicExcellent gap filling/planarizing characteristicOrganic chemistrySemiconductor/solid-state device manufacturingOrganic filmBenzene
Owner:SHIN ETSU CHEM IND CO LTD +1
C-H fluorination of heterocycles with silver (II) fluoride
Owner:RGT UNIV OF CALIFORNIA
Pesticide composition for preventing and treating soil insects
InactiveCN103858929AGood killing effectGood control effectBiocideNematocidesThiazoleRoot-knot nematode
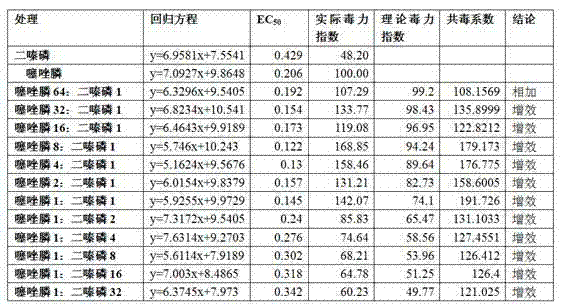
The invention discloses a pesticide composition for preventing and treating soil insects. The pesticide composition comprises following effective components: thiazole phosphine and diazinon, wherein the mass ratio of the thiazole phosphine to the diazinon is (64:1)-(1:32). Prepared from the thiazole phosphine and the diazinon in a compounding manner, the pesticide composition has good compatibility and a quite good synergistic interaction effect, and has the advantages of obviously improving the prevention and treatment effect on root-knot nematode and grub, reducing the total amount of used pesticide, lowering the use cost and also effectively preventing and treating soil insects including mole cricket, agrotis ypsilon and the like.
Owner:ZHEJIANG SHIYUAN JINNIU PESTICIDE CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com