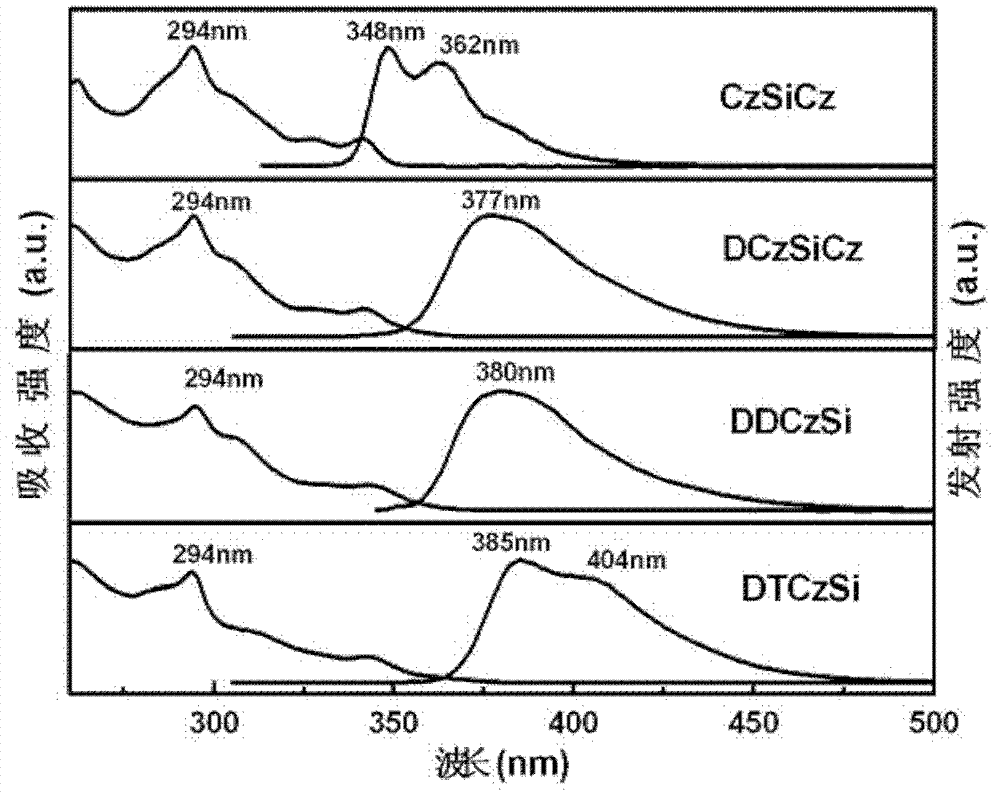
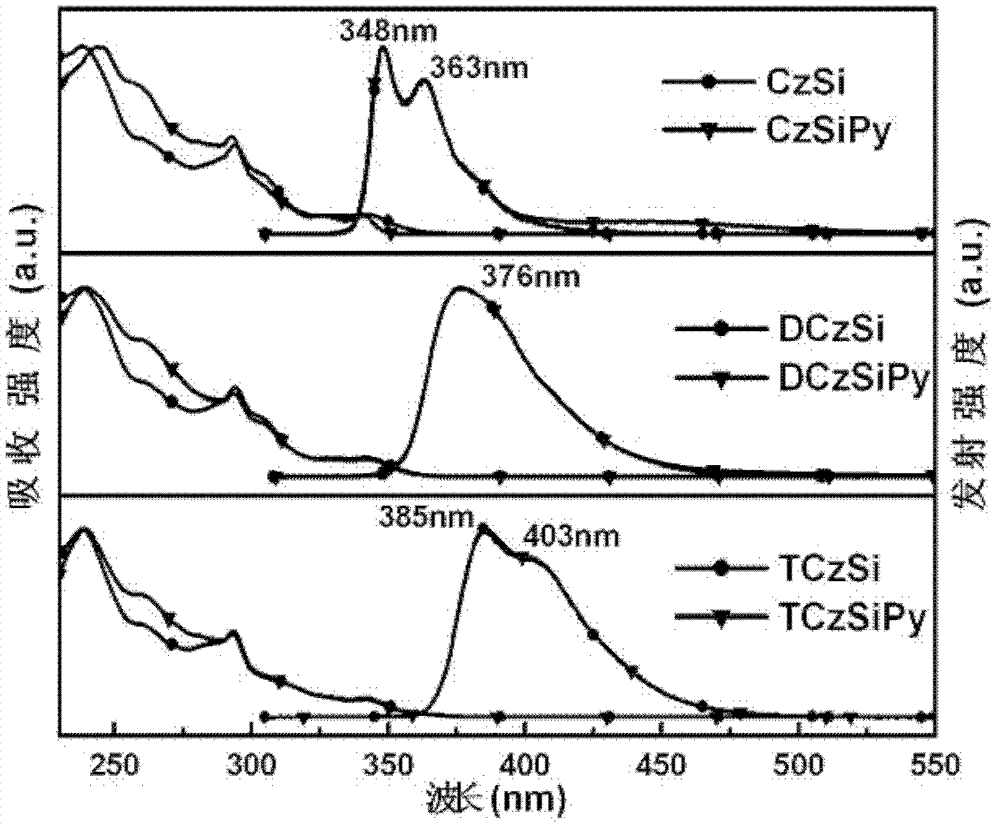
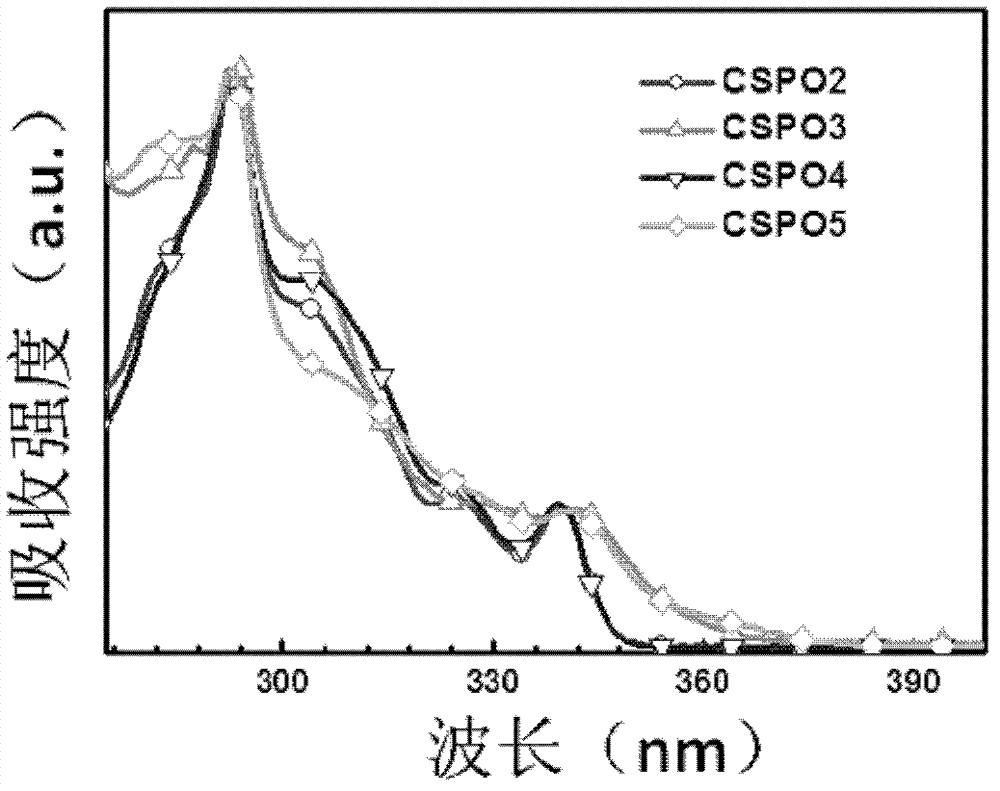
Phosphorescent dye parent materials with tetrahedral structure, and application thereof in electroluminescent device
A phosphorescent dye, tetrahedral technology, applied in the field of organic electroluminescent materials, can solve the problem of lack of parent materials, etc., and achieve the effects of high carrier migration ability and high electroluminescence efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0035] Synthesis of SiPy
[0036]
[0037] Combine 0.83g (2mmol) 4-bromophenyl-triphenyl silicon, 0.30g (2.4mmol) 4-pyridineboronic acid and 92mg (4mol%) [(PPh 3 ) 4 The Pd(0) catalyst was dissolved in a mixed solvent of 12mL toluene, 6mL methanol and 6mL sodium carbonate solution with a concentration of 2mol / L. The system was degassed for three times and then transferred to an oil bath and reacted at 85°C for 48 hours. The reaction solution was poured into water, extracted three times with dichloromethane, and the collected organic phase was dried with anhydrous magnesium sulfate. After filtration, use a rotary evaporator to concentrate the solution, use a mixed solvent of dichloromethane, petroleum ether and ethyl acetate (volume ratio 1:4:1) as the eluent, and purify by thin layer chromatography to obtain 0.68g of white solid , The yield is 82%. 1 H NMR(CDCl 3 , 500MHz, ppm): δ8.67-8.66 (2H, d, J = 4.27 Hz, Ar-H), 7.71-7.69 (2H, d, J = 7.94 Hz, Ar-H), 7.65-7.64 (2H, d, J = 8....
Synthetic example 2
[0039] Synthesis of CzSi
[0040]
[0041] 0.84g (5mmol) of carbazole, 2.49g (6mmol) of monomer 4-bromophenyl-triphenylsilica, 95mg (10mol%) of Cul catalyst, 114mg (20mol%) of trans 1,2- Diaminocyclohexane, 2.23g (210mol%) potassium phosphate, 12mL toluene solvent were placed in a 100mL round bottom flask, the system was degassed three times and then transferred to an oil bath, and reacted at 110°C for 48 hours. The reaction solution was poured into water, extracted three times with dichloromethane, and the collected organic phase was dried with anhydrous magnesium sulfate. After filtration, the solution was concentrated with a rotary evaporator, using a mixed solvent of dichloromethane and petroleum ether (volume ratio of 1:6) as the eluent, thin layer chromatography to obtain 1.23 g of white solid with a yield of 49%. 1 H NMR(CDCl 3 , 500MHz, ppm): δ8.16-8.14 (2H, d, J=7.94Hz, Ar-H), 7.82-7.81 (2H, d, J=8.24Hz, Ar-H), 7.67-7.65 (6H, m, Ar-H), 7.62-7.60 (2H, d, J=8.24 Hz, Ar-H)...
Synthetic example 3
[0043] Synthesis of DCzSi
[0044]
[0045] The synthesis process is the same as CzSi. White solid, the yield is 62%. 1 H NMR(CDCl 3 , 500MHz, ppm): δ 8.29-8.28 (1H, d, J=1.83 Hz, Ar-H), 8.19-8.18 (2H, d, J=7.94 Hz, Ar-H), 8.12-8.10 (1H, d, J = 7.63 Hz, Ar-H), 7.88-7.86 (2H, d, J = 8.24 Hz, Ar-H), 7.69-7.66 (9H, m, Ar-H), 7.57-7.54 (2H, m , Ar-H), 7.51-7.43 (10H, m, Ar-H), 7.41-7.39 (4H, m, Ar-H), 7.34-7.28 (3H, m, Ar-H), 13 C NMR(CDCl 3 , 125MHz, ppm): 142.29, 141.74, 140.16, 139.11, 138.47, 136.86, 134.64, 134.21, 130.46, 130.30, 128.49, 127.10, 126.60, 126.28, 125.93, 124.96, 123.54, 121.00, 120.69, 120.02, 119.91, 111.44, 110.72, 110.23. Theoretical value of element analysis: C 48 H 34 N 2 Si: C, 86.45%; H, 5.14%; N, 4.20%. Actual value: C, 86.71%; H, 5.08%; N, 4.16%. Mass spectrum: m / z 667.3 (100%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com