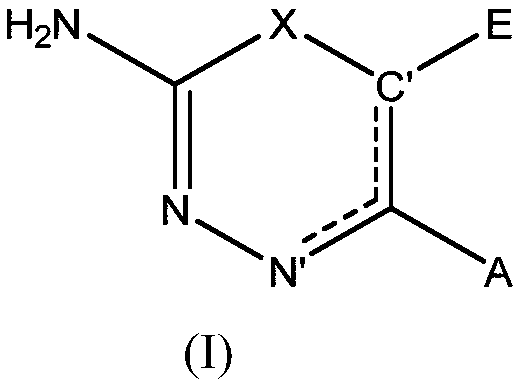
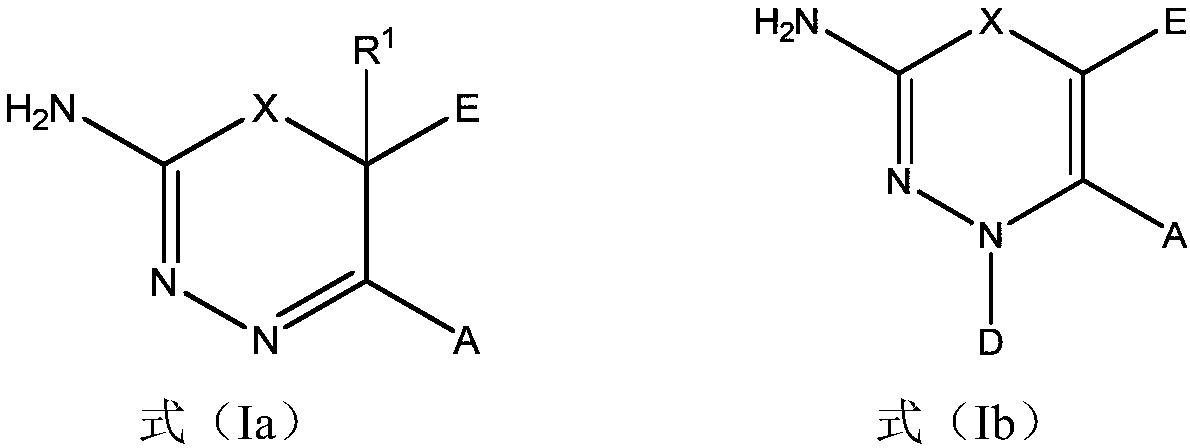
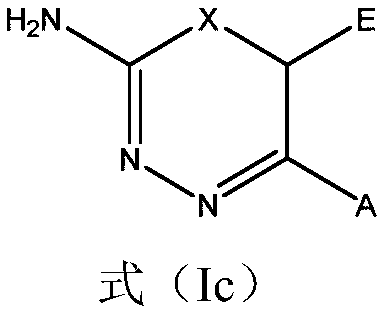
2-amino-1,3,4-thiadiazine and 2-amino-1,3,4-oxadiazine based antifungal agents
A technology of antifungal agents and diazines, which can be applied in the direction of antifungal agents, medical preparations containing active ingredients, active ingredients of heterocyclic compounds, etc., and can solve the problems of flucytosine’s limited therapeutic use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0220] Example 1: 5-(2-Methoxy-ethyl)-6H-[1,3,4]thiadiazin-2-ylamine
[0221]
[0222] A) Preparation of 1-chloro-4-methoxy-butan-2-one.
[0223] At 0°C, to anhydrous AlCl 3 (4.87g, 36.6mmol) dissolved in 50mL of anhydrous CH 2 Cl 2 To the resulting solution was added dropwise 3-chloro-2-ethoxymethoxy-propene (5.0 g, 36.6 mmol) in CH 2 Cl 2 solution, and the reaction mixture was stirred at room temperature for 2 hours. The reaction was monitored by TLC. After the reaction was complete, the reaction mixture was quenched with 200 mL of ice water, and then the mixture was subjected to standard ether treatment to gradually reach the desired product yield of 4 g (80%). The product was a brown liquid, which was obtained as crude The product is used in the next step.
[0224] B) Preparation of 5-(2-methoxy-ethyl)-6H-[1,3,4]thiadiazin-2-ylamine.
[0225] To a solution of 1-chloro-4-methoxybutan-2-one (4.0 g, 29.3 mmol) dissolved in 40 mL of acetonitrile was added thiosemicar...
Embodiment 2
[0226] Example 2: 5-m-tolyl-6H-[1,3,4]thiadiazin-2-ylamine hydrobromide
[0227]
[0228] To a solution of 3-methylphenacyl bromide (1.0 g, 4.3 mmol) in ethanol (20 mL) was added thiosemicarbazide (400 mg, 4.3 mmol) at 0°C, and after the addition was complete, the mixture was allowed to warm to room temperature and stir overnight. The resulting slurry was cooled to -20°C and the precipitate was collected by filtration, washed with cold ethanol and dried in vacuo. The pale yellow solid was suspended in 20 mL of ethanol containing 1 mL of 48% aqueous hydrobromic acid. The mixture was heated to reflux for 30 minutes, then cooled to room temperature overnight. The precipitate was filtered and purified by silica gel column chromatography using hexane:EtOAc (49:1) to afford 950 mg (70%) of 5-m-tolyl-6H-[1,3,4]thiadiazine-2- amine hydrobromide.
Embodiment 3
[0229] Example 3: 5-(3-Chloro-phenyl)-6H-[1,3,4]thiadiazin-2-ylamine hydrobromide
[0230]
[0231] A) Preparation of 2-bromo-1-(3-chloro-phenyl)-ethanone.
[0232] To a stirred solution of 1-(3-chloro-phenyl)-ethanone (5.0 g, 32.3 mmol) in chloroform (75 mL) was added bromine (1.66 mL, 32.2 mmol) at 5-10 °C. After the addition was complete, the temperature of the reaction mixture was slowly raised to room temperature and stirred at room temperature for 1 hour. The reaction was monitored by TLC, after the reaction was completed, the reaction was washed with 10% Na 2 S 2 o 3 solution (100 mL), the reaction mixture was diluted with CH 2 Cl 2 (50 mL) for extraction. The organic layer was washed with water (400 mL), dried and concentrated to afford 7.2 g (96%) of crude 2-bromo-1-(3-chloro-phenyl)-ethanone, which was used without purification in the following one step.
[0233] B) Preparation of 5-(3-chloro-phenyl)-6H-[1,3,4]thiadiazin-2-ylamine hydrobromide
[0234] To...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com