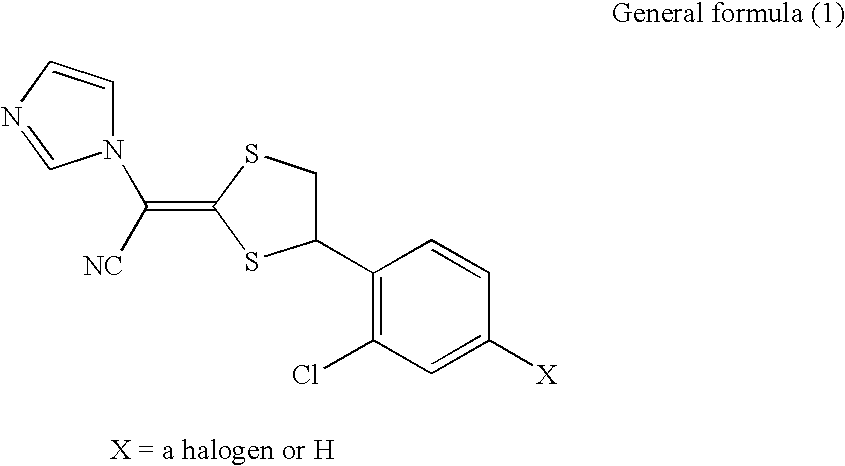
Pharmaceutical composition
a pharmaceutical composition and composition technology, applied in the field of skin agents, can solve the problems of not having a definite law for maintaining not knowing how to achieve the maintenance and not having a definite law for the maintenance property of the steric structur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0030]Luliconazole was dissolved by adding an appropriate amount of ethanol. To the resulting solution, POE (20) sorbitan monostearate (“NIKKOL TS-10MV”; manufactured by Nihon Surfactant Kogyo K.K.) was gradually added, water was further added, and homogenization was performed (Table 1). The resultant was named as Skin agent A for external use. Further, Skin agent B for external use as a control was prepared in the same manner as in Skin agent A for external use except that ethanol was added in place of water.
TABLE 1Prescription of Skin agent A for external useIngredientmass %Luliconazole1.2POE (20) sorbitan monostearate7.5Water23.3Ethanol68Total100
[0031]Form of formulation: a form of a single-phase and homogeneously dissolved solution was confirmed through a visual check.
No irritating sensation of the formulation was confirmed.
[0032]For Skin agent A for external use and Skin agent B for external use, the time-dependent stability of luliconazole was evaluated 1 week after preparatio...
example 2
[0033]In the same manner as in Example 1, luliconazole was dissolved in ethanol. To the resulting solution, POE (20) sorbitan monooleate (“NIKKOL TO-10MV”; manufactured by Nihon Surfactant Kogyo K.K.) was gradually added, water was further added, and homogenization was performed to prepare Skin agent C for external use. Further, Skin agent D for external use as a control was prepared in the same manner as in Skin agent C for external use except that ethanol was added in place of water.
TABLE 3Prescription of Skin agent C for external useIngredientmass %Luliconazole1.2POE (20) sorbitan monooleate9.3Water23.3Ethanol66.2Total100
[0034]Form of formulation: a form of a single-phase and homogeneously dissolved solution was confirmed through a visual check.
No irritating sensation of the formulation was confirmed.
[0035]For Skin agent C for external use and Skin agent D for external use, the time-dependent stability of luliconazole was evaluated 1 week after preparation under a preservation co...
example 3
[0036]In the same manner as in Example 1, luliconazole was dissolved in ethanol. To the resulting solution, polyoxyethylene hydrogenated castor oil 40 (“NIKKOL HCO-40”; manufactured by Nihon Surfactant Kogyo K. K.) was gradually added, water was further added, and homogenization was performed to prepare Skin agent E for external use. Further, Skin agent F for external use as a control was prepared in the same manner as in Skin agent E for external use except that ethanol was added in place of water.
TABLE 5Prescription of Skin agent E for external useIngredientmass %Luliconazole1.2Polyoxyethylene hydrogenated1.4castor oil 40Water23.7Ethanol73.7Total100
[0037]Form of formulation: a form of a single-phase and homogeneously dissolved solution was confirmed through a visual check.
No irritating sensation of the formulation was confirmed.
[0038]For Skin agent E for external use and Skin agent F for external use, the time-dependent stability of luliconazole was evaluated 1 week after preparat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass % | aaaaa | aaaaa |
| mass % | aaaaa | aaaaa |
| mass % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com