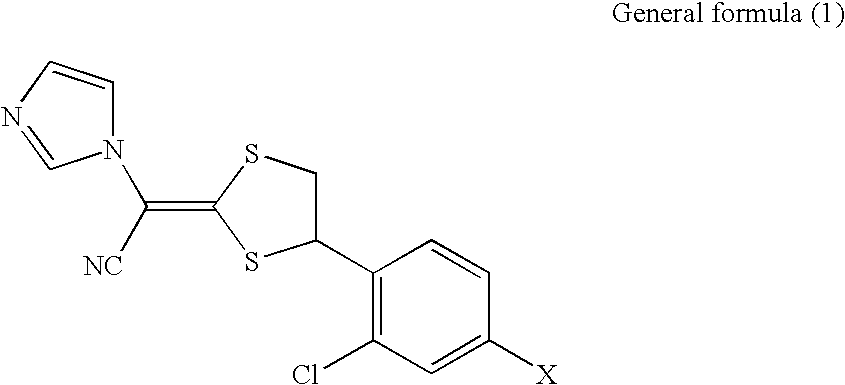
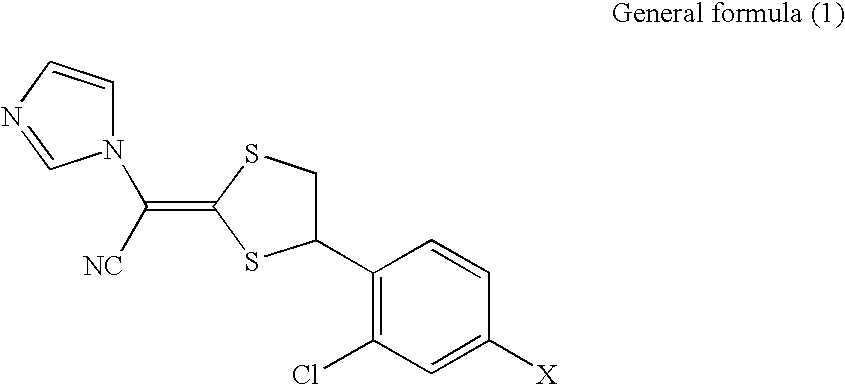
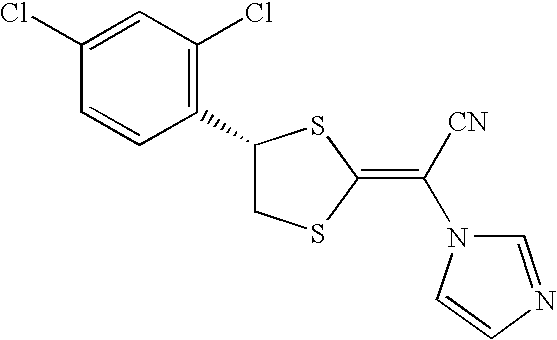
Antifungal composition
a technology of composition and antifungal agent, applied in the field of pharmaceutical composition, can solve the problems of (i) poor solubility of compounds represented by the general formula in an aqueous medium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0035]Skin agents 1 to 5 for external use, each of which is the pharmaceutical composition of the present invention, were prepared in accordance with the following prescription. That is, luliconazole was dissolved by adding an appropriate amount of ethanol, and to the resulting solution, triacetin and glucono-δ-lactone were added and uniformly dispersed. After that, hydroxypropylcellulose was gradually added, and water was further added to obtain homogenous skin agents for external use. Any of those agents had a viscosity of 100 to 800 mPa·s (viscometer: RE80R manufactured by Toki Sangyo Co., Ltd., and conditions for viscosity measurement: cone angle; 0.8°, cone radius; 2.4 cm, rotation frequency; 10 rpm, and temperature; 25° C.), was not so dripping, and thus was suited as a formulation for seborrheic dermatitis to be administered to the hair line and the like. Further, those agents were free of ingredients strongly exhibiting solvent characteristics likemethyl ethyl ketone (MEK) a...
example 2
[0043]In the same manner as in Example 1, Skin agent 6 for external use as the pharmaceutical composition of the present invention was prepared in accordance with the following prescription. The concentration of luliconazole in the horny cell layer at 2 hours after the application of the agent was 4.532 μg / cm2, and the concentration in Comparative Example 1 in which propylene glycol in the preparation was replaced by ethanol was 0.221 μg / cm2. The S-E isomer measured by HPLC after harsh preservation (60° C., 1 week) was 1.65% in Skin agent 6 for external use, which was higher compared with 1.45% in Comparative Example 1. Propylene glycol is found to be usable as an ingredient for promoting the absorption to the horny cell layer.
TABLE 6Ingredientw / v %Luliconazole1Propylene glycol50EthanolAppropriate amountTotal100 mL
example 3
[0044]In the same manner as in Example 1, Skin agent 7 for external use as the pharmaceutical composition of the present invention was prepared in accordance with the following prescription. The concentration of luliconazole in the horny cell layer at 2 hours after the application of the agent was 0.584 μg / cm2, and the concentration in Comparative Example 2 in which 2-ethyl-1,3-hexanediol in the preparation was replaced by ethanol was 0.950 μg / cm2. The S-E isomer measured by HPLC after harsh preservation (60° C., 1 week) was 1.06% in Skin agent 7 for external use, which was higher compared with 0.38% in Comparative Example 2. 2-ethyl-1,3-hexanediol is found to be usable as an ingredient for inhibiting the absorption to the horny cell layer.
TABLE 7Ingredientw / v %Luliconazole1Hydroxypropylcellulose1.5Water202-ethyl-1,3-hexanediol5EthanolAppropriate amountTotal100 mL
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com