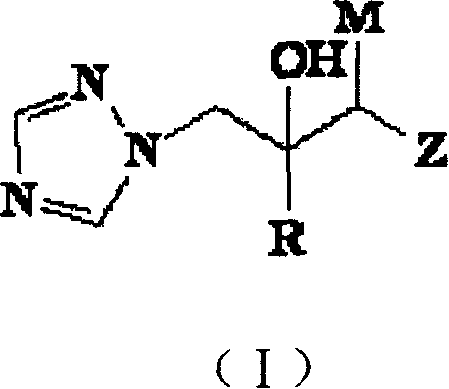
Antifungal compound of alkyl substitutional triazole class
A technology of triazoles and antifungal, which is applied in the field of medicine and can solve the problems of increased difficulty in synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Synthesis of 1-(1H-1,2,4-triazol-1-yl)-2-tert-butyl-3-(2-pyridylmercapto)-2-propanol
[0079] Take 1.78g (16mmol) 2-mercaptopyridine, 4.15g (15mmol) 1-(1H-1,2,4-triazol-1-yl)-2-tert-butyl-2,3-epoxypropylene methanesulfonate salt and 5.52g (40mmol) of potassium carbonate were put into a 100ml three-necked flask, then a little tetrabutylammonium bromide and 55ml DMF were added, stirred and reacted at 75°C for 8h, filtered, and the filtrate was adjusted to pH=2 with hydrochloric acid, ethyl acetate The ester was washed (30ml×3), the water phase was adjusted to pH=10 with 10% sodium hydroxide, the solid was filtered out, and recrystallized with absolute ethanol to obtain 1.4g of light yellow powder, yield 31.96%, mp: 68-70°C .
[0080] 1 H-NMR (DMSO-d 6 )δ, ppm: 8.44 (1H, s, triazole C 3 -H), 7.91 (1H, s, triazole C 5 -H), 8.32 (1H, d, pyridine-C 6 -H), 7.62 (1H, m, pyridine-C 4 -H), 7.28 (1H, d, pyridine-C 3 -H), 7.12 (1H, m, pyridine-C 5 -H), 5.50 (1H, s, OH), 4....
Embodiment 2
[0083] 1-(1H-1,2,4-triazol-1-yl)-2-tert-butyl-3-[4-(4-methoxyphenyl)piperazin-1-yl]-2-propane Alcohol synthesis
[0084] Add 1.39g (5mmol) 1-(1H-1,2,4-triazol-1-yl)-2-tert-butyl-2,3-epoxypropylene methanesulfonate successively in a 100ml three-necked flask, 0.96g (5mmol) 1-[4-(4-methoxy)phenyl]piperazine, 1.38g (10mmol) potassium carbonate, a little cetyltrimethylammonium bromide and 30ml absolute ethanol, at 90 Heat and stir in a water bath at ℃ for 7 hours, pour into 100ml of water, extract with ethyl acetate (30ml×3), wash with water, dry over anhydrous sodium sulfate, recover the solvent to obtain a solid, recrystallize with ethyl acetate to obtain 0.83g of white needle crystals , Yield: 55.63%, mp: 110-111°C.
[0085] 1 H-NMR (DMSO-d 6 )δ, ppm: 7.94, 8.48 (2H, s, s, triazole-H), 6.77~6.84 (4H, m, Ar-H), 4.53 (1H, s, OH), 4.32 (2H, s, -CH 2 -), 3.67 (3H, s, -OCH 3 ), 2.29-2.88 (10H, m, piperazine-H,-CH 2 -), 0.91(9H, s, -C(CH 3 ) 3 )
[0086] IR (cm -1 , KBr): 3...
Embodiment 3
[0088] 1-(1H-1,2,4-triazol-1-yl)-2-tert-butyl-3-[4-(4-ethoxyphenyl)piperazin-1-yl]-2-propane Alcohol synthesis
[0089] Add 1.39g (5mmol) 1-(1H-1,2,4-triazol-1-yl)-2-tert-butyl-2,3-epoxypropylene methanesulfonate successively in a 100ml three-necked flask, 1.03g (5mmol) 1-[4-(4-ethoxy)phenyl]piperazine, 1.38g (10mmol) potassium carbonate, a little cetyltrimethylammonium bromide and 30ml absolute ethanol, according to implementation According to the operation steps of Example 2, 1.17 g of white crystals were obtained, yield: 60.5%, mp: 78-80°C.
[0090] 1 H-NMR (DMSO-d 6 )δ, ppm: 7.94, 848 (2H, s, s, triazole-H), 6.76~6.82 (4H, m, Ar-H), 4.52 (1H, s, OH), 4.32 (2H, s, -CH 2 -), 3.93 (2H, q, J=7.0Hz, -OCH 2 -), 2.27-2.88 (10H, m, piperazine-H, -CH 2 -), 1.28 (3H, t, J=70Hz, -CH 3 ), 0.91 (9H, s, -C (CH 3 ) 3 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com