High-volume composite hydrogen storage material, and synthetic method and hydrogen desorption method thereof
A hydrogen storage material and high-capacity technology, applied in chemical instruments and methods, hydrogen, hydrogen production, etc., can solve the problems of high hydrogen depletion temperature, unfavorable practical application of materials, slow hydrogen desorption kinetics, etc., and achieve simple synthesis process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. LiBH 4 -NH 2 NH 2 Preparation of complex
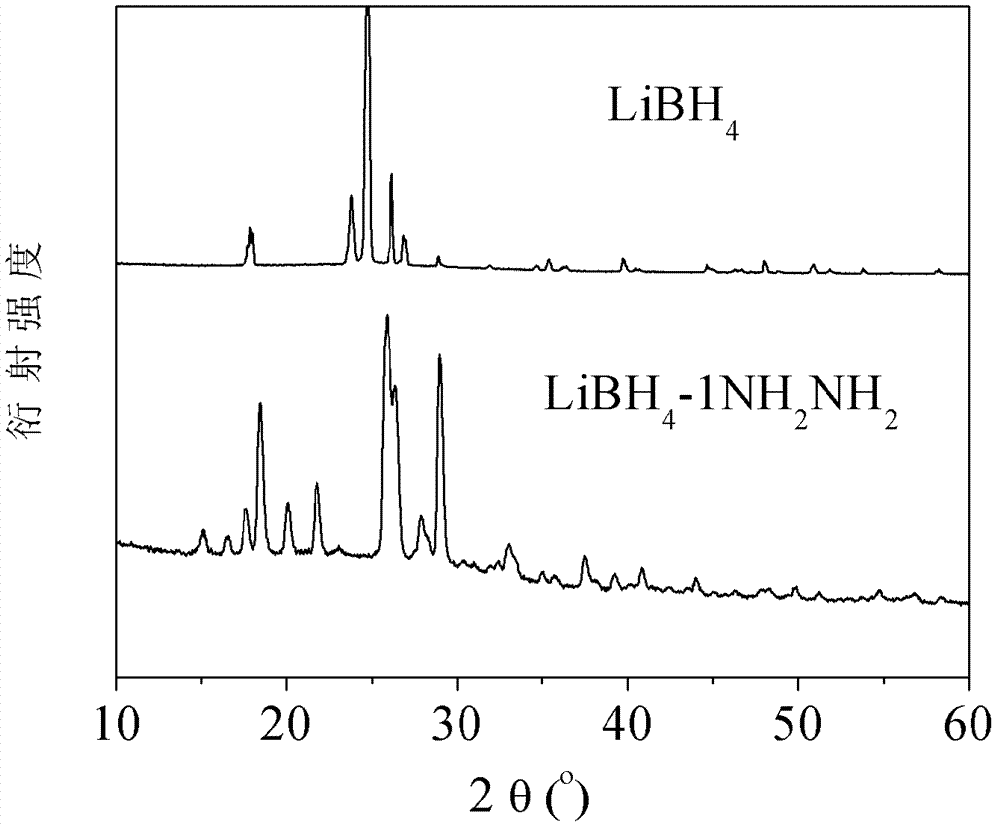
[0039] Weigh 361.8 mg of lithium borohydride and 0.5 ml of hydrazine in the glove box, and put the two samples into the same ball mill jar. Note that the two samples cannot be in contact at this time. After the ball mill jar was sealed, it was carefully transferred to a ball mill, and ball milled for 2 hours at 150 rpm. After the ball milling, the pressure in the ball milling tank did not change, and the prepared sample was solid. figure 1 For the X-ray diffraction (XRD) spectrogram of the prepared sample, it can be seen that, with pure LiBH 4 Compared to LiBH 4 -NH 2 NH 2 The complex forms a new species, completely different from LiBH 4 .
[0040] 2. Addition of catalyst
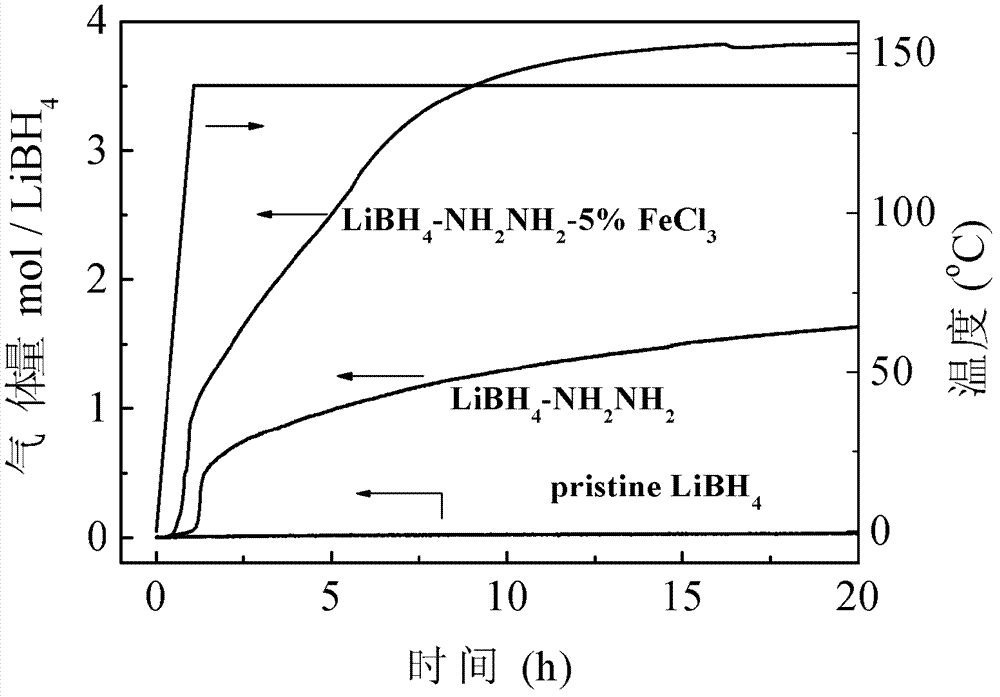
[0041] Weigh 500mg of LiBH in the glove box 4 -NH 2 NH 2 complex, while weighing 77.6mg of FeCl 3 , put the two samples into the same ball mill jar. After sealing the ball mill jar, carefully transfer it to a ball mill, and mill it for 5 h...
Embodiment 2
[0045] 1. Mg(BH 4 ) 2 -2NH 2 NH 2 Preparation of complex
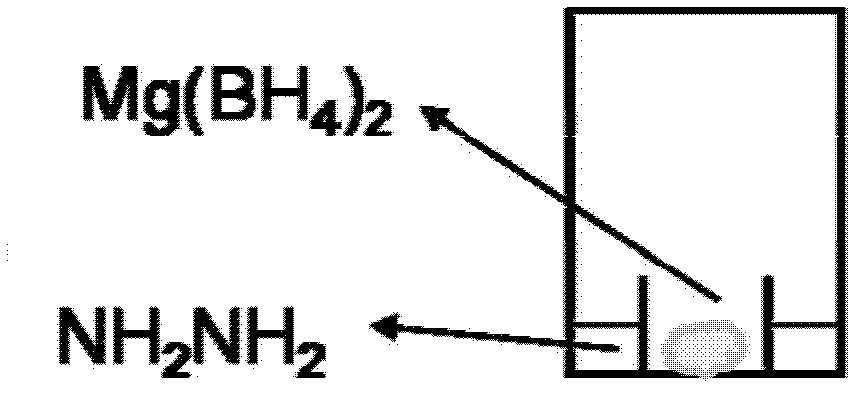
[0046] Weigh 444.1mg of homemade Mg (BH 4 ) 2 , and measure 0.5ml of hydrazine at the same time, put the two samples into image 3 in the airtight container shown in , without touching each other. Using the vapor pressure of hydrazine, Mg(BH 4 ) 2 Hydrazine can be completely adsorbed into the solid phase to form Mg(BH 4 ) 2 -2NH 2 NH 2 Complex. Sample Mg(BH 4 ) 2 -2NH 2 NH 2 Must be aged in a closed system for 1 week.
[0047] 2. Dehydrogenation reaction
[0048] Weigh Mg(BH 4 ) 2 -2NH 2 NH 2 100 mg of the sample was placed in a closed reaction tube, starting from room temperature, the temperature was programmed to rise to 250°C at 2°C / min, and the temperature was kept at 250°C until the end of the reaction. Such as Figure 4 As shown, the measured hydrogen release amount of the system is about 7.6 equiv.H 2 / Mg(BH 4 ) 2 , corresponding to 12.8 wt% H 2 / Mg(BH 4 ) 2 -2NH 2 NH 2 .
Embodiment 3
[0050] LiH-NH 2 NH 2 preparation of
[0051] Weigh 128.9 mg of lithium hydride and 0.5 ml of hydrazine in the glove box, and put the two samples into the same ball mill jar. Note that the two samples cannot be in contact at this time. After the ball mill jar was sealed, it was carefully transferred to a ball mill, and ball milled for 2 hours at 150 rpm. After the ball milling, the gas in the ball mill tank was measured by mass spectrometry to be hydrogen, and the pressure change was 1mol H 2 / LiH, and the prepared sample is solid. Figure 5 For the X-ray diffraction (XRD) spectrogram of the prepared sample, it can be seen that compared with pure LiH, LiH and NH 2 NH 2 The reaction produces a new species, completely different from LiH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com