In-situ preparation method of nanometer magnesium hydride
An in-situ preparation of magnesium hydride technology, applied in the field of hydrogen storage materials and nanomaterials, can solve the problems of unfavorable applications and reduce the hydrogen storage capacity of materials, and achieve the effect of maintaining capacity, high hydrogen storage capacity and avoiding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1 nanometer magnesium hydride
[0059] (1) Put 500mg of anhydrous magnesium chloride and 500mg of lithium hydride in an argon atmosphere glove box, put them into ball mill jars respectively, and ball mill them at a speed of 350 rpm for 3 hours.
[0060] (2) In an argon atmosphere glove box, take a total of 600mg of ball-milled anhydrous magnesium chloride and lithium hydride (molar ratio 1:2), put it into a 250ml flask, then inject 150ml of ultra-dry tetrahydrofuran into the flask, and stir for 30min , to be fully dissolved magnesium chloride.
[0061] (3) Insert the ultrasonic rod into the flask, keep the end of the ultrasonic rod in the middle of the mixture in the flask, and perform ultrasonic treatment with an output power of 210W. During the ultrasonic process, in order to keep the temperature of the sample from rising too high, continuous ultrasonic for half an hour must be intermittent for half an hour , accumulatively processed for...
Embodiment 2
[0080] The preparation of the nano-magnesium hydride sample of embodiment 2Ti catalytic doping
[0081] (1) Anhydrous magnesium chloride (500 mg) and lithium hydride (500 mg) were placed in a ball mill jar respectively in an argon atmosphere glove box, and ball milled at a speed of 350 rpm for 3 hours.
[0082] (2) In an argon atmosphere glove box, take a total of 600 mg of ball-milled anhydrous magnesium chloride and lithium hydride (molar ratio 1:2) and titanium tetrachloride (TiCl 4 ) (16.4 μL), put into a 250ml flask, then inject 100ml ultra-dry cyclohexane into the flask and stir for 30min, the ratio of the present embodiment, the molar ratio of Ti catalyst to magnesium chloride is 0.03:1.
[0083] (3) Insert the ultrasonic rod into the flask, keep the end of the ultrasonic rod in the middle of the mixture in the flask, and perform ultrasonic treatment with an output power of 210W. During the ultrasonic process, in order to keep the temperature of the sample from rising t...
Embodiment 3
[0099] The preparation of the nano-magnesium hydride sample of embodiment 3V catalytic doping
[0100] The same as the preparation method of Example 2, the difference is that the transition metal catalyst used is: vanadium trichloride VCl 3 , Table 1 lists the corresponding raw material ratio and key process, the sample named nano-MgH 2 -0.03V.
[0101] Table 1 nano-MgH 2 -0.03V sample preparation process and raw material ratio
[0102] Ultrasonic power (W) Ultrasonic time (h) MgCl 2 +LiH(mg)
VCl 3 (mg)
210 6 600 24
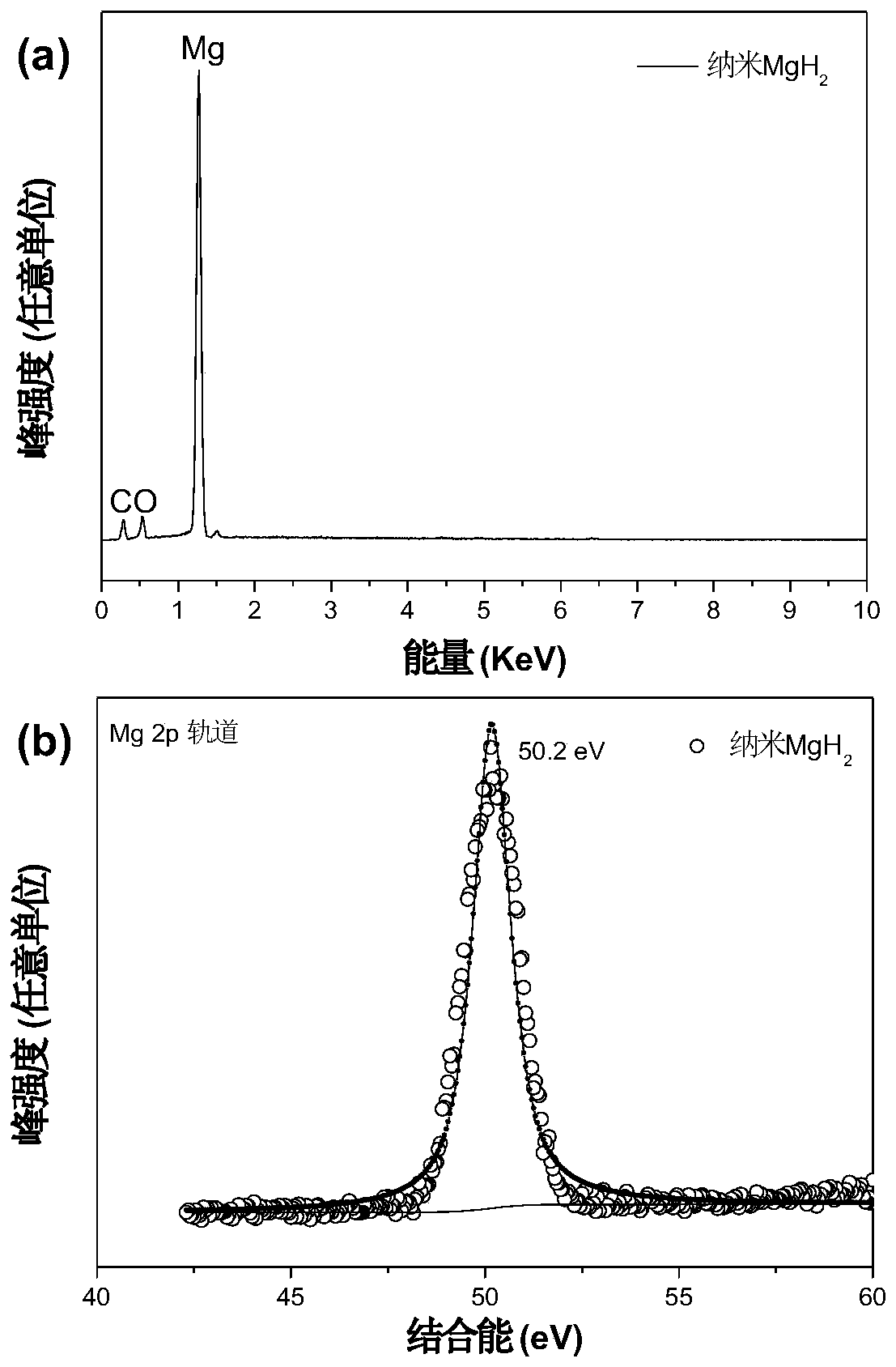
[0103] From Figure 12 In a, it can be seen that the sample only contains magnesium and vanadium elements. The weak oxygen signal and carbon signal come from carbon and oxygen pollution in the air or a small amount of organic solvent residue, indicating that the main phase of the sample is magnesium hydride, and a small amount of vanadium is used as a catalyst. ;From Figure 12 It can be seen in b that the V2p of the titan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com