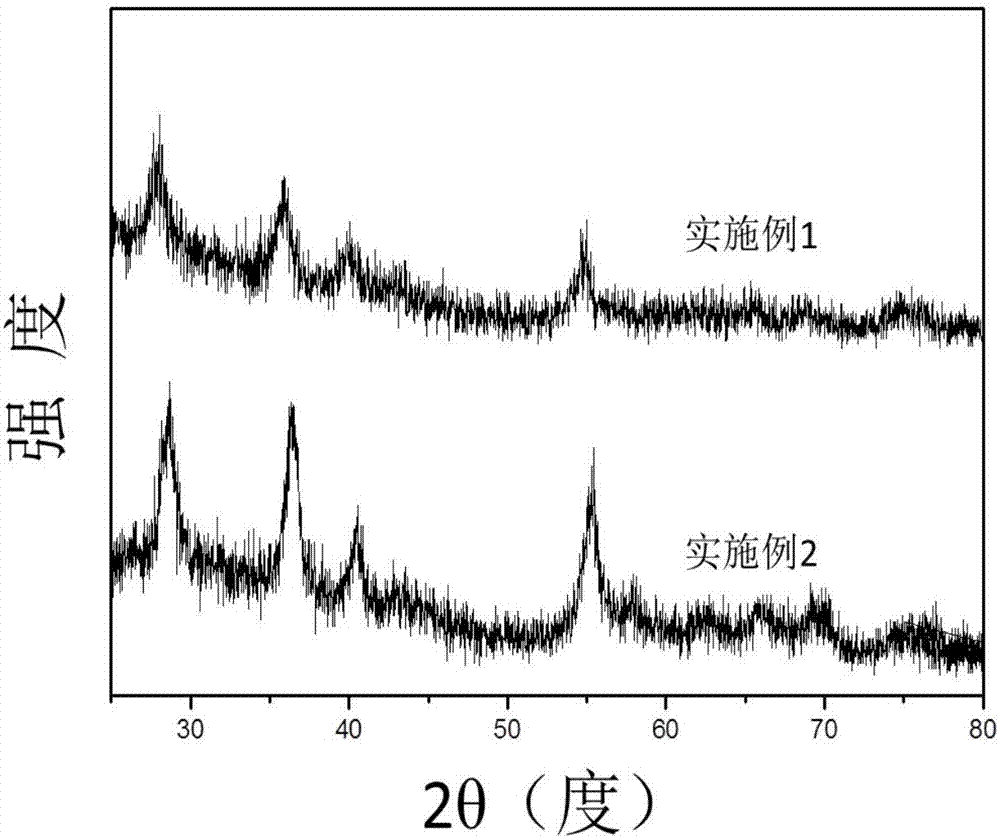
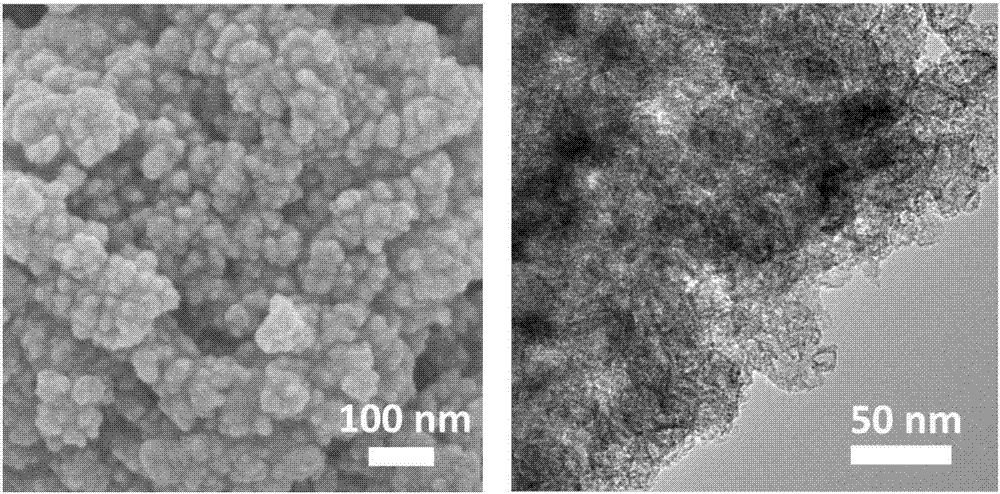
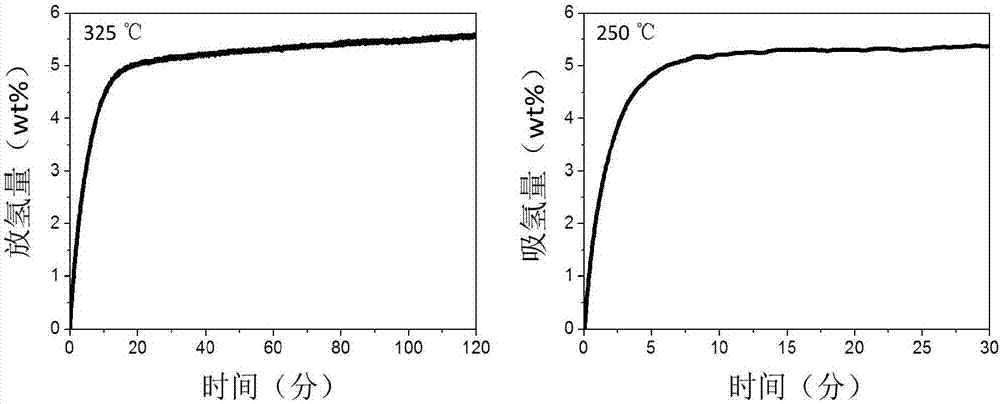
Patents
Literature
200 results about "Magnesium hydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
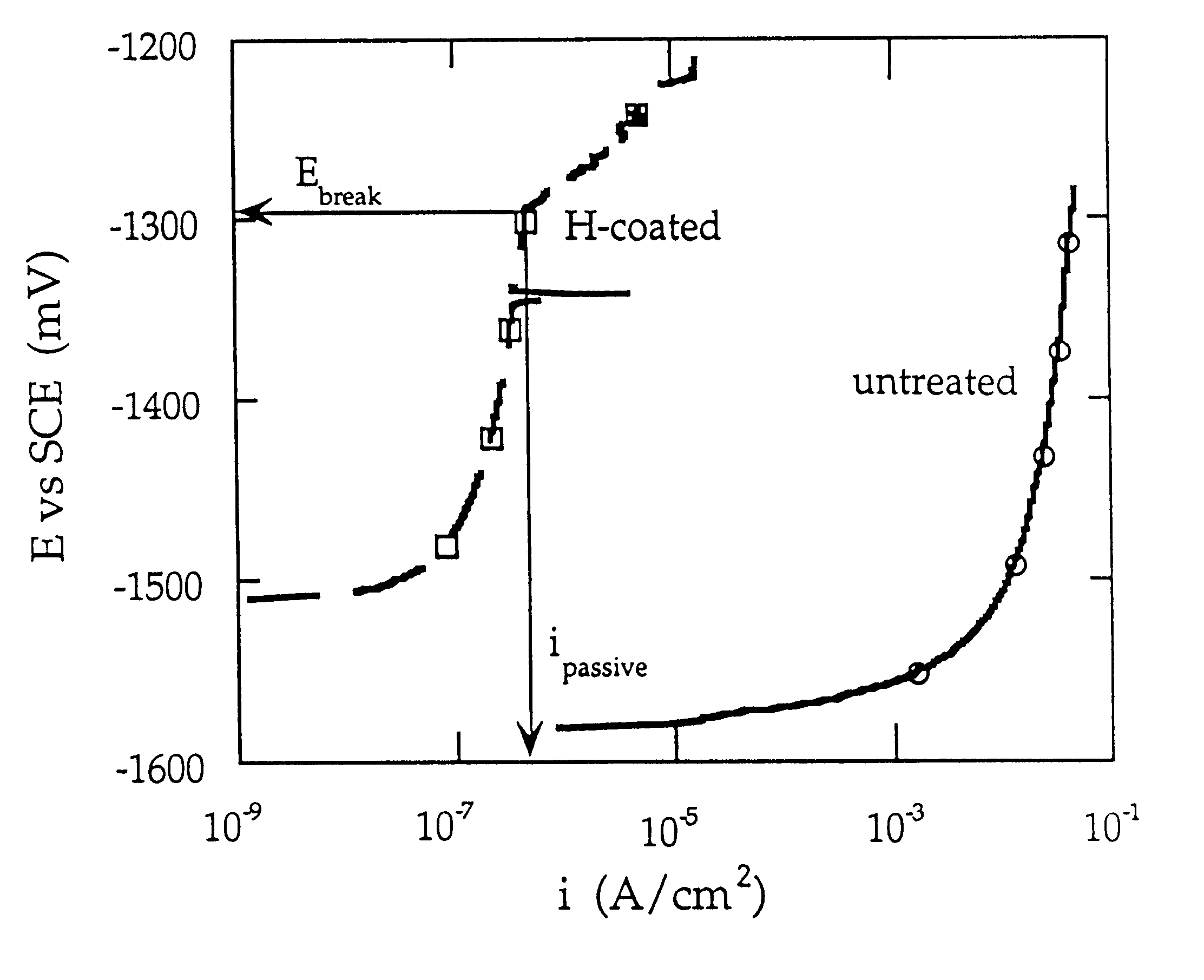
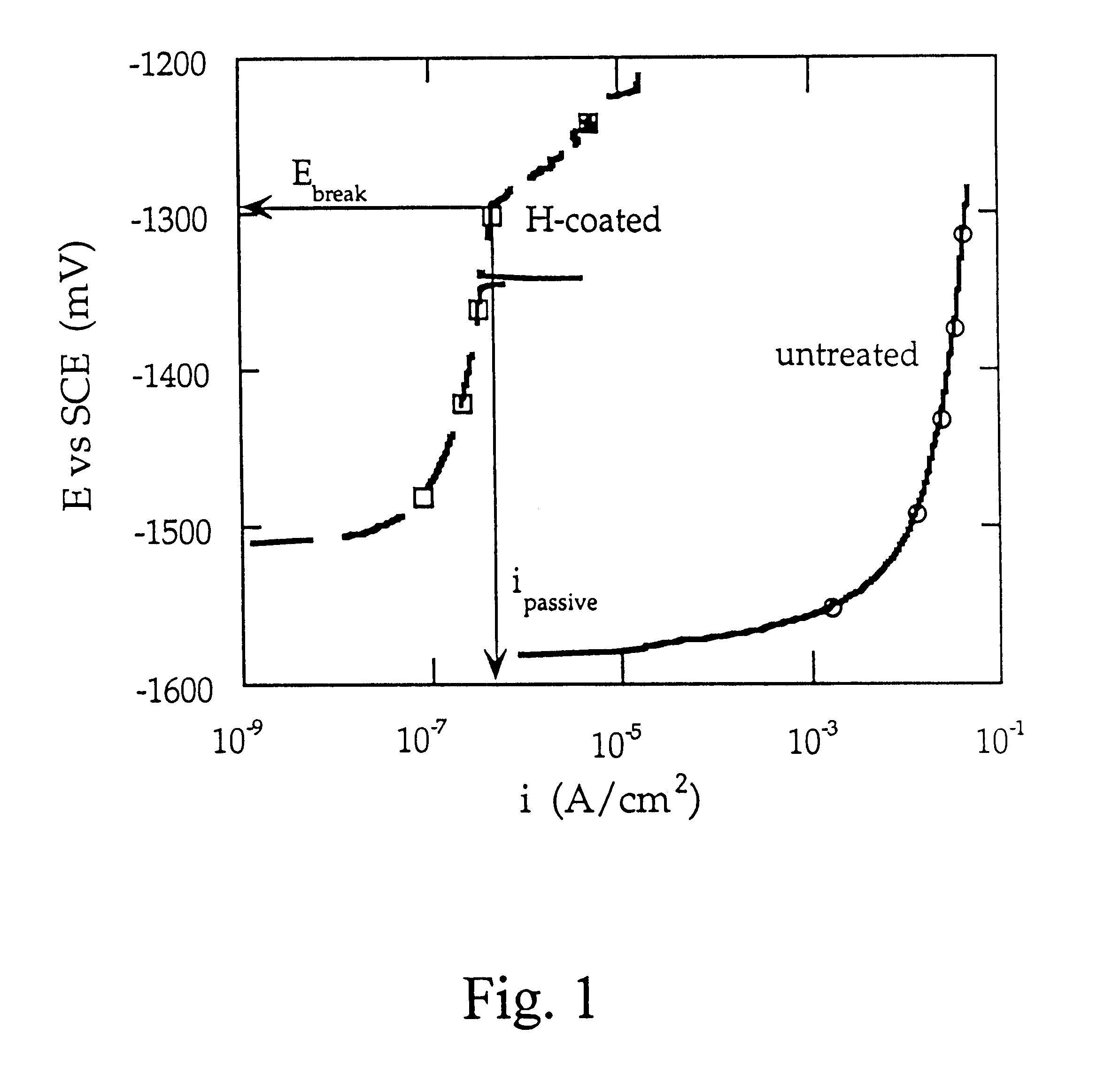
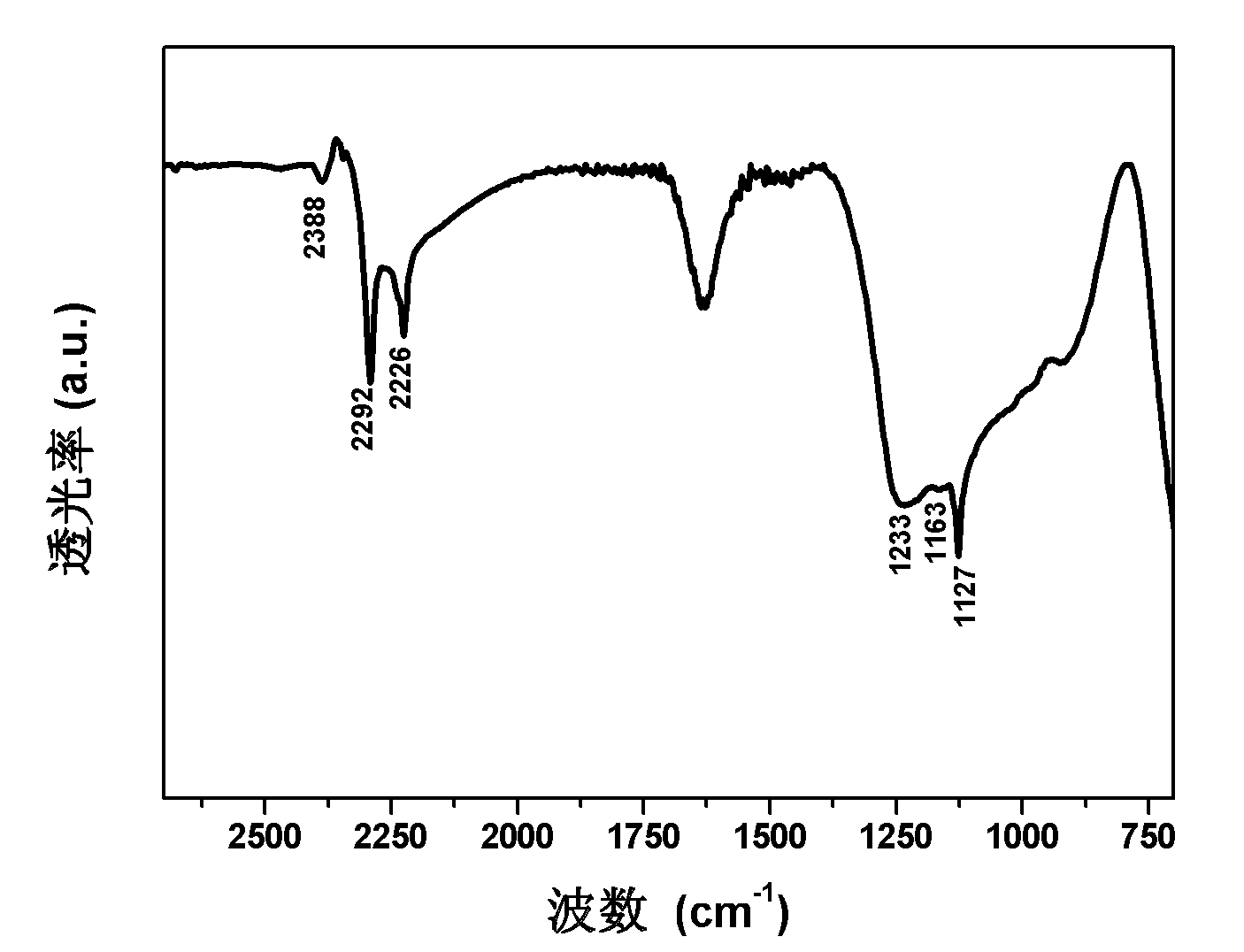
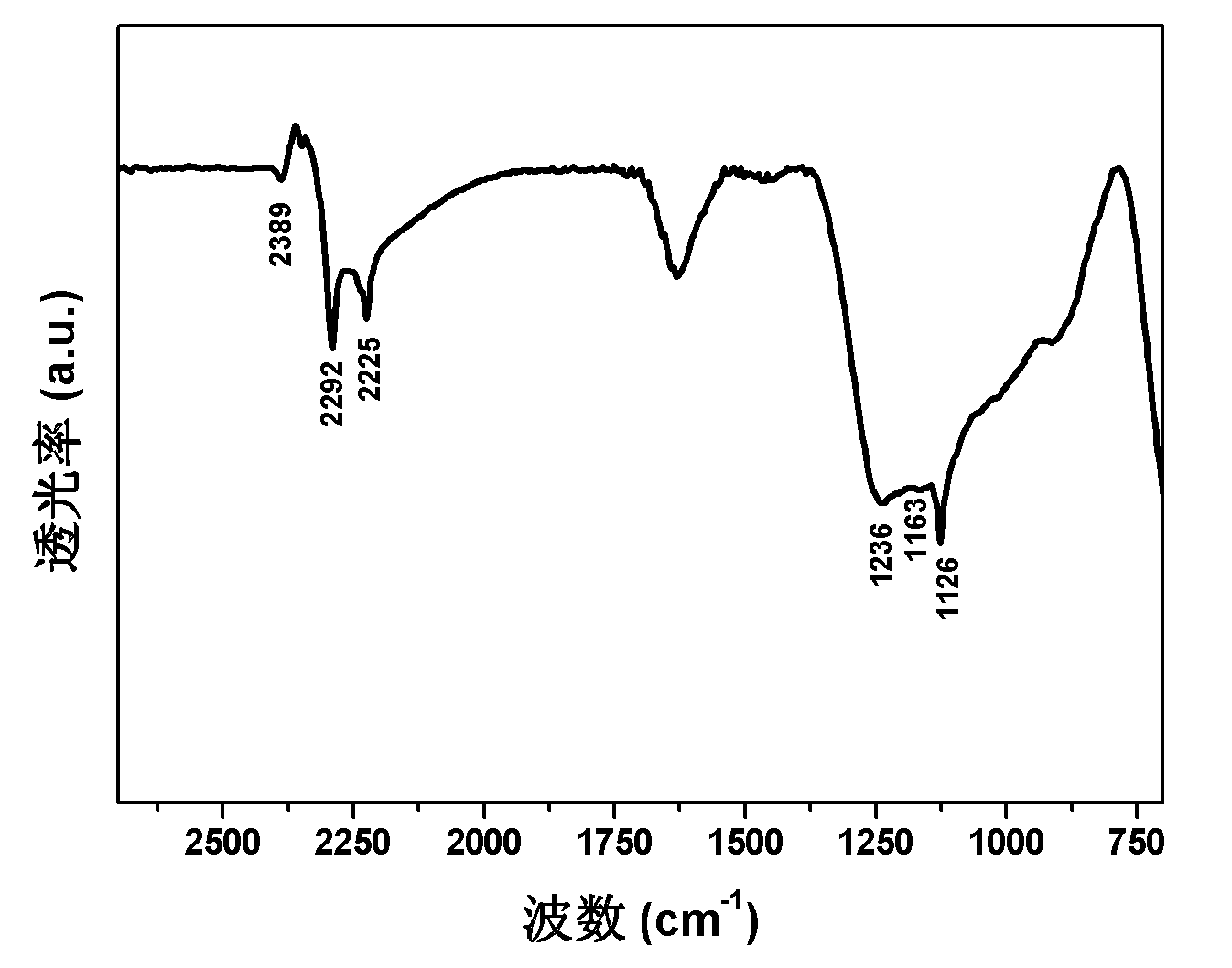
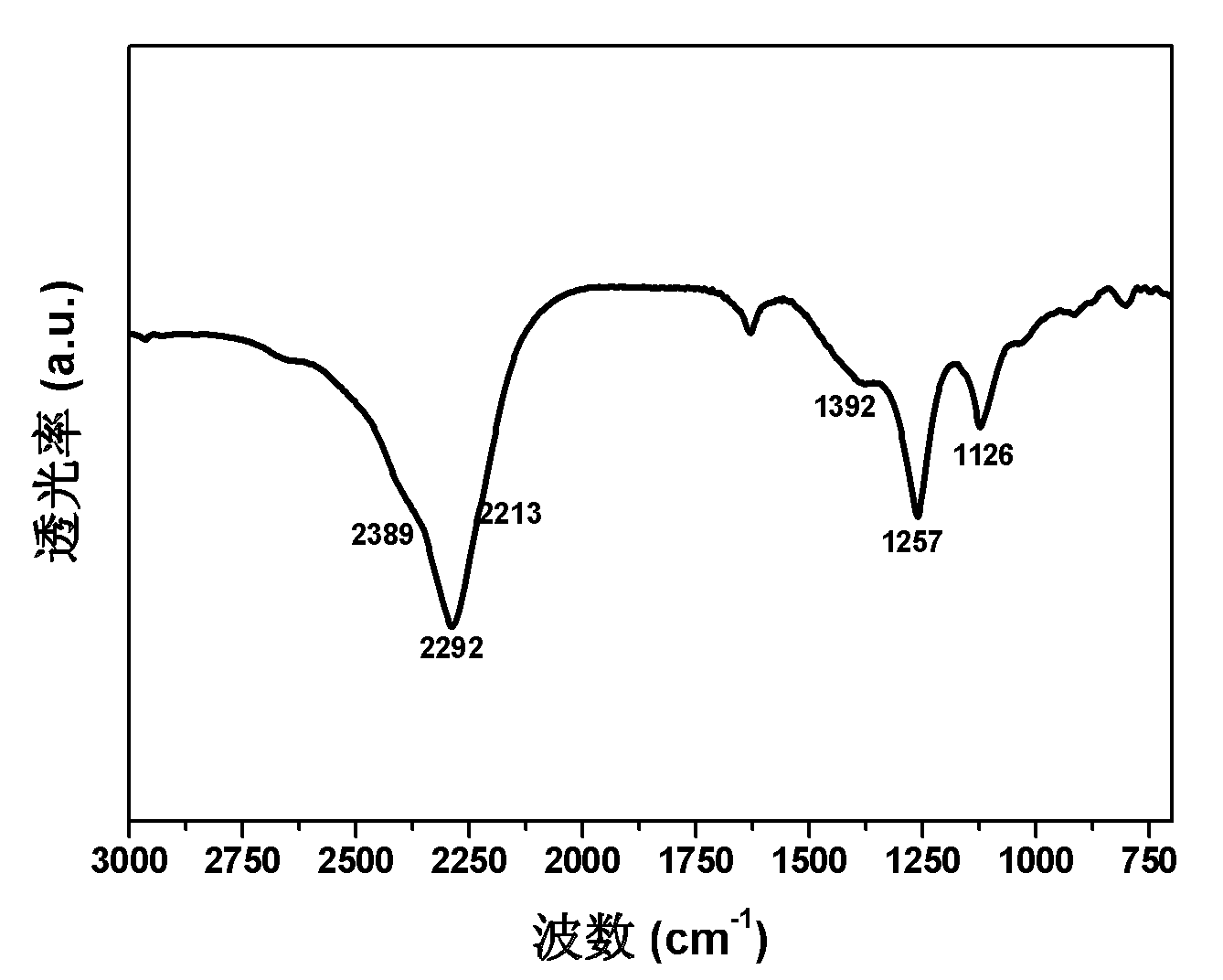
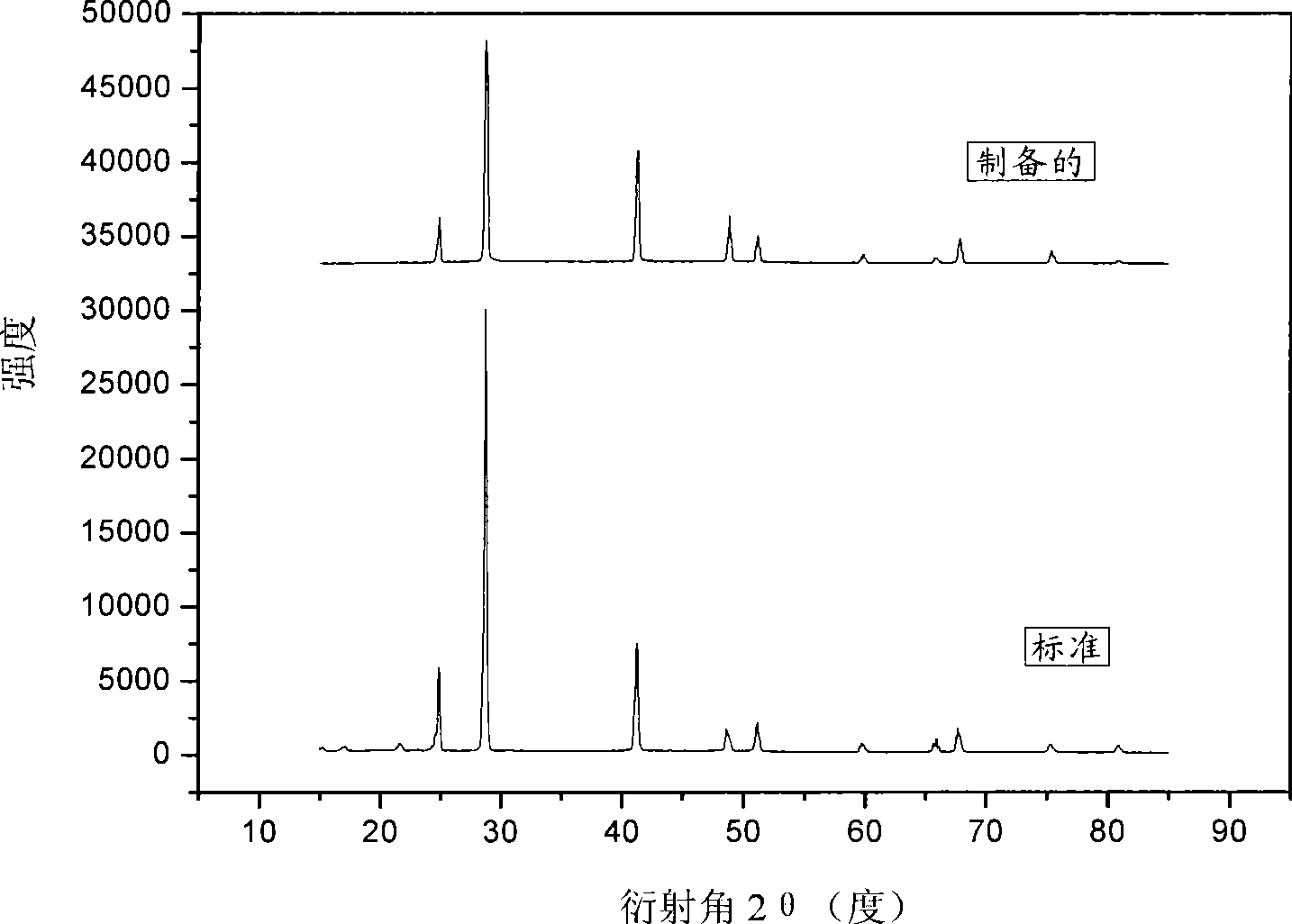
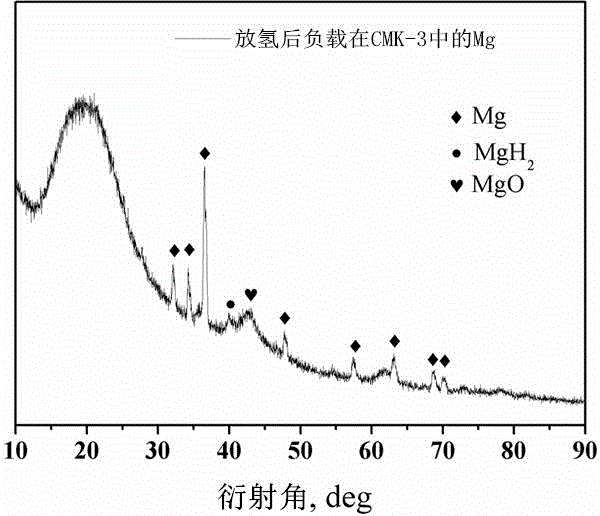
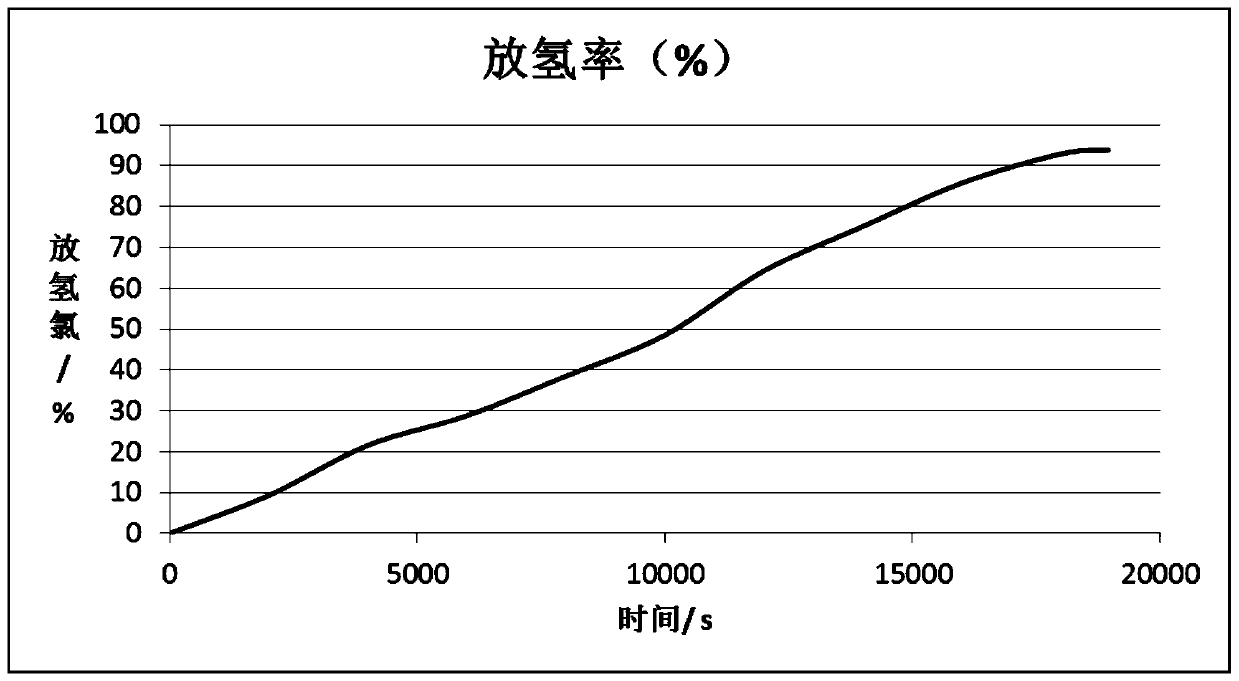
Magnesium hydride is the chemical compound with the molecular formula MgH₂. It contains 7.66% by weight of hydrogen and has been studied as a potential hydrogen storage medium.
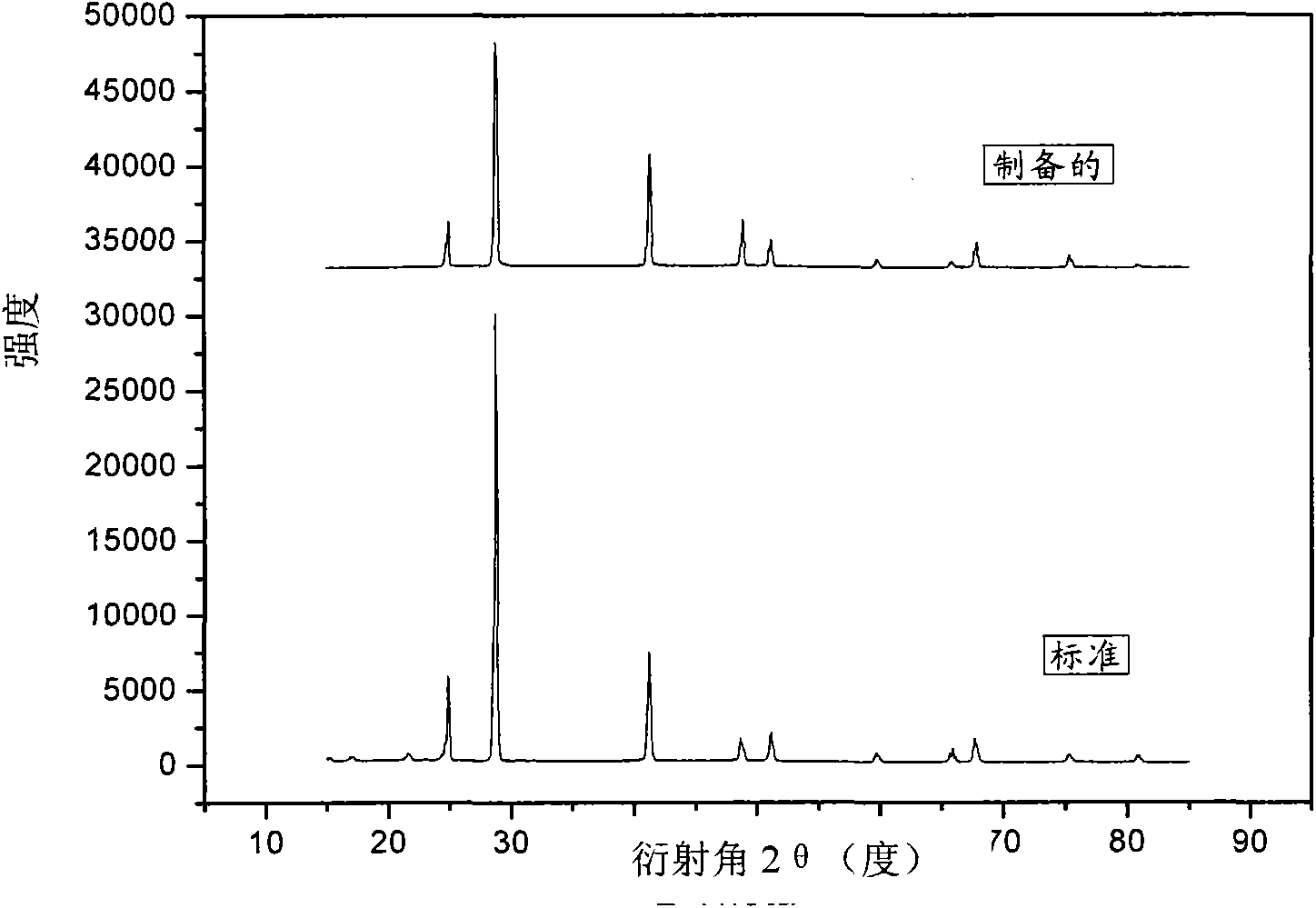
Method for preparing sodium borohydride by recycling sodium metaborate
The invention relates to a method for preparing alkali metals hydroboron, in particular to a method for preparing sodium borohydride by recycling sodium metaborate, which solves the problem of recycling a byproduct sodium metaborate after the sodium borohydride is hydrolyzed to prepare hydrogen in the prior art, and is a novel method for preparing the sodium borohydride. The method takes magnesiumhydride as a hydrogen source and the sodium metaborate as a boron source, and prepares the sodium borohydride through mechanical and chemical reaction. In the presence of the hydrogen, magnesium powder is hydrogenated at certain temperature and pressure to prepare magnesium hydride; and the sodium metaborate and the magnesium hydride in a certain ratio are put into a ball mill, certain argon or hydrogen pressure, ratio of grinding media to material and grinding time are maintained, and the sodium metaborate can be reduced into the sodium borohydride. The method has the advantages of simple, convenient and safe process, no environmental pollution and the like.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Magnesium hydride nano particle and preparation method and application thereof
InactiveCN101117211ALarge specific surface areaShorten the diffusion distanceAlkali/alkaline-earth/beryllium/magnesium hydridesHydrogen absorptionNanotechnology
The invention discloses a magnesium hydride nanometer granular material and the preparing method and application, wherein nanometer magnesium powder is prepared through adopting arc heating method and then the magnesium powder is hydrogenated to obtain MgH2 granular product with the grain diameter being 50 nm to 600 nm. The nanometer MgH2 grain possesses high purity and outstanding kinetics property of hydrogen absorption and desorption during being used as hydrogen storage material, thereby possessing extremely important application value and extensive application potential in the hydrogen storage technical field.
Owner:PEKING UNIV
Cathodic protective coating on magnesium or its alloys
InactiveUS6291076B1Simple and efficientConvenient and economical solutionAnodisationNatural mineral layered productsHydrogenAlloy
A method is provided for treating a magnesium-containing article to form a cathodic protective coating on such article. This is done by electrochemically treating the article, acting as a cathode, in an alkaline solution, preferably at a temperature of between 40 and 80° C., with a cathodic current density of 5-200 mA / cm2. The treatment produces a magnesium-containing article having a protective coating of magnesium hydride of predetermined thickness with a high count of hydrogen particles.
Owner:INTERMAG MODELEX
Nano composite hydrogen-storing material and preparing method
InactiveCN1743066ASimple preparation processImprove hydrogen storage performanceHydrogenAlkali/alkaline-earth/beryllium/magnesium hydridesNano carbonNanometre
The present invention provides a nano composite hydrogen storage material. It is a mixture obtained by mixing 90 wt%-99 wt% of magnesium hydride and 1 wt%-10 wt% of nano carbon. Its preparation method includes the following steps: mixing nano carbon into magnesium hydride according to the required ratio, under the atmosphere of argon gas or hydrogen gas mechanically ball-grinding for 30 min-100 hr, then making desorption; or ball-grinding magnesium hydride for 1-100 hr, then adding nano carbon and ball-grinding for 30 min-10 hr, then making desorption. Its hydrogen storage capacity is 4.5 wt%-6.7 wt%.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Composite hydrogen storage material contg. magnesium-transition metals oxides, prepn. method and application thereof
InactiveCN1903423AHigh hydrogen storage capacityImproved hydrogen storage kineticsAlkali/alkaline-earth/beryllium/magnesium hydridesOther chemical processesHigh energyHydrogen fuel cell
Owner:NANKAI UNIV
Method of Manufacturing Pure Titanium Hydride Powder and Alloyed Titanium Hydride Powders By Combined Hydrogen-Magnesium Reduction of Metal Halides
ActiveUS20130315773A1Reduce oxideFacilitated DiffusionMultiple metal hydridesTransition element hydridesTitanium chloridePowder mixture
The invention relates to energy-saving manufacturing of purified hydrogenated titanium powders or alloying titanium hydride powders, by metallo-thermic reduction of titanium chlorides, including their hydrogenation, vacuum separation of titanium hydride sponge block from magnesium and magnesium chlorides, followed by crushing, grinding, and sintering of said block without need for hydrometallurgical treatment of the produced powders.Methods disclosed contain embodiments of processes for manufacturing high-purity powders and their use in manufacturing near-net shape titanium and titanium-alloy articles by sintering titanium hydride and alloyed titanium hydride powders produced from combined hydrogen-magnesium reduction of titanium chlorides, halides and hydrides of other metals. Additional titanium hydride powder introduced with titanium tetrachloride beneficially affects the kinetics of magnesium-thermic reduction due to formation of the additionally-emitted atomic hydrogen, which helps to reduce presence of oxides and so cleans inter-particle interfaces of the product and enhances diffusion between all of components of the powder mixture.
Owner:ADVANCED MATERIALS PRODS
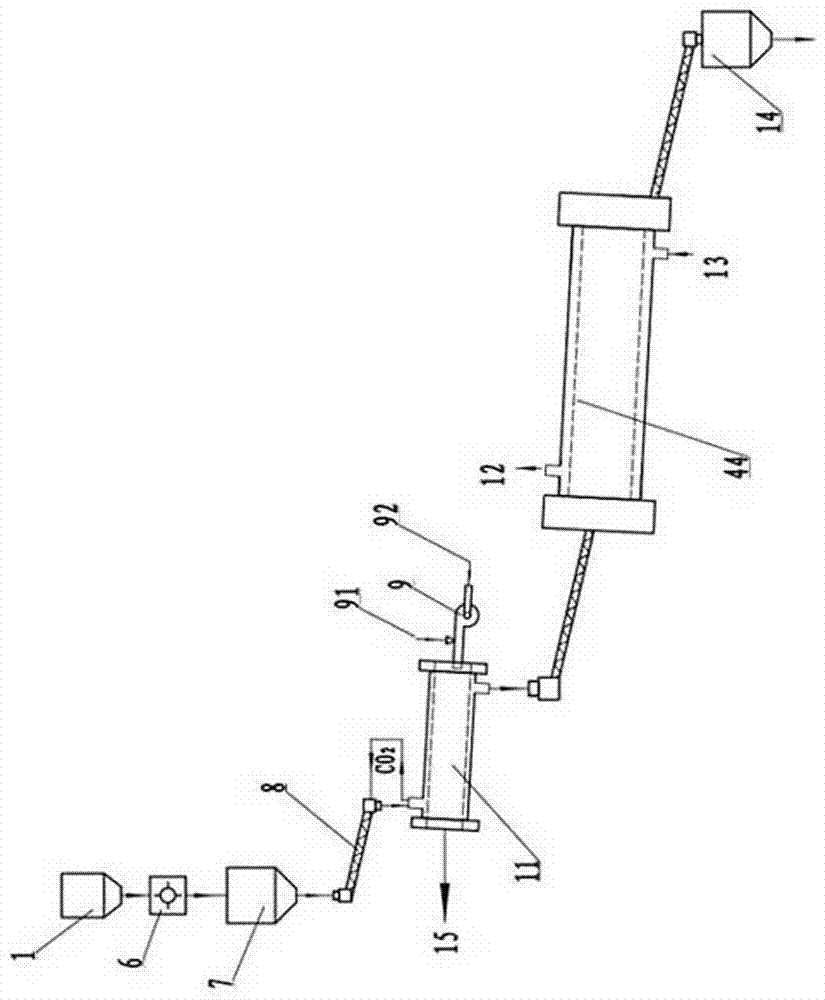
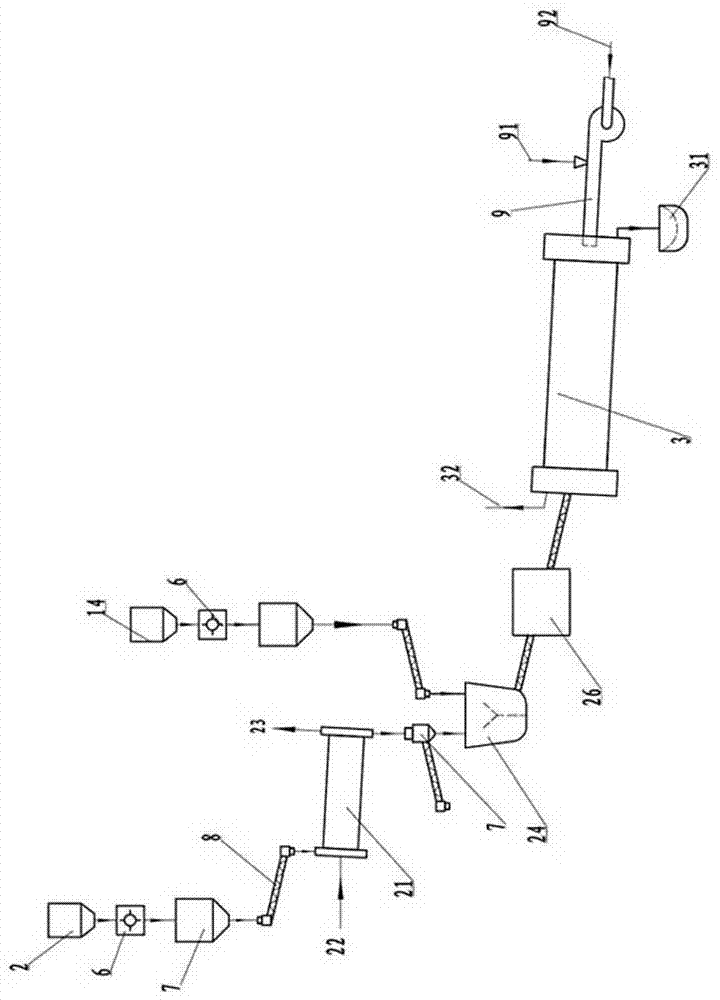
Magnesium hydride type hydrogen-stored emulsion explosive
The invention belongs to the technical field of explosive preparation, in particular to a magnesium hydride type hydrogen-stored emulsion explosive. The components comprise an emulsion base and a sensitized material, wherein the sensitized material is magnesium hydride; the mass of the magnesium hydride takes up 0.2-6% of the total mass of the emulsion explosive; the magnesium hydride is powders with purity not smaller than 95%. The added magnesium hydride is uniformly distributed in the emulsion base such that a part of magnesium hydride has a chemical reaction with water in the emulsion base, and a little amount of hydrogen is released, and foaming effect is performed on the emulsion base, and the detonator sensitivity is improved. When the emulsion base of the mixed magnesium hydride is initiated, the hydrogen bubbles under the function of shock waves produced in emulsion base detonation generate detonation reaction, and at the same time, the rest magnesium hydride quickly releaseshydrogen under the denotation waves of the emulsion base, and the hydrogen joins the detonation reaction of the emulsion base such that the emulsion explosive has characteristics of a high output shock wave peak value, a high impulse and a high energy. The detonation energy of the emulsion explosive is better than that of the common emulsion explosive.
Owner:UNIV OF SCI & TECH OF CHINA
Calcium-magnesium hydrogen producing agent
InactiveCN102783691AOut-of-the-boxStrong reducing agentCosmetic preparationsToilet preparationsWrinkle skinSkin elasticity
The invention discloses a calcium-magnesium hydrogen producing agent, of which the main component is a hydrogen producing agent. The hydrogen producing agent is selected from one or various combination of calcium hydride, magnesium hydride, calcium hydroxide magnesium, magnesium-magnesium hydride, calcium-calcium hydride, magnesium-calcium hydride, calcium-magnesium hydride, calcium-magnesium-magnesium hydride, calcium-magnesium-calcium hydride, calcium-magnesium-magnesium hydride-calcium hydride, coral calcium hydroxide magnesium, Na MgH3, and Mg2FeH6. the calcium-magnesium hydrogen producing agent can be added into water, beverage or wine as an additive, or can be coated on the surface of skin or taken as soaking liquid to remove internal or surface active oxygen. The calcium-magnesium hydrogen producing agent has a remarkable prompting effect for recovering various oxidized damages, relieving discomfort caused by drinking, diluting stain and wrinkle of the skin, recovering skin elasticity, and delaying senescence.
Owner:李志林
Solid-phase synthesis method of Mg(BH4)2 hydrogen storage material
InactiveCN102730639ANo pollution in the processEasy to operateMonoborane/diborane hydridesHydrogen productionHigh pressure hydrogenHigh pressure
The invention discloses a solid-phase synthesis method of a Mg(BH4)2 hydrogen storage material. Magnesium boride or a mixture of magnesium boride and magnesium hydride is used as a starting material to carry out a mechanochemical reaction in high pressure hydrogen atmosphere, with a catalyst selected from one or more of a transition metal, a transition metal halide, a transition metal hydride and a transition metal oxide, so as to prepare the Mg(BH4)2. The preparation method of the Mg(BH4)2 hydrogen storage material of the invention has advantages of simple synthetic method, easily controlled reaction process, low cost and no organic pollution.
Owner:ZHEJIANG UNIV
Method for preparing sodium borohydride by chemical mechanical mechanics method
InactiveCN101519188ALow costSimple and safe processMonoborane/diborane hydridesChemical reactionHydrogen pressure
The invention relates to a method for preparing alkali borohydride, in particular to a method for preparing sodium borohydride by a chemical mechanical mechanics method and solving the problems of environmental pollution, severe operation condition and the like during the preparation process of the sodium borohdride in the prior art. The method takes magnesium hydride as a hydrogen source, takes borax as a boron source and prepares the sodium borohydride by combining mechanical grinding with chemical reaction. In the hydrogen atmosphere, under a certain temperature and pressure, the magnesium powder is mechanically grinded and generates hydrogenation reaction with the hydrogen, thus preparing magnesium hydride; subsequently, the borax, sodium carbonate and magnesium hydride are arranged in a ball mill with a certain proportion; certain hydrogen pressure, ball material ratio and grinding time are kept, thus obtaining the sodium borohydride. The purity of the sodium borohydride prepared by the method can achieve more than 97%. The chemical mechanical mechanics method is adopted to prepare the sodium borohydride, and compared with the currently general Schlesinger method and Baeyer method, the method has the advantages of low raw material cost, simple, convenient and safe process, no pollution and the like.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
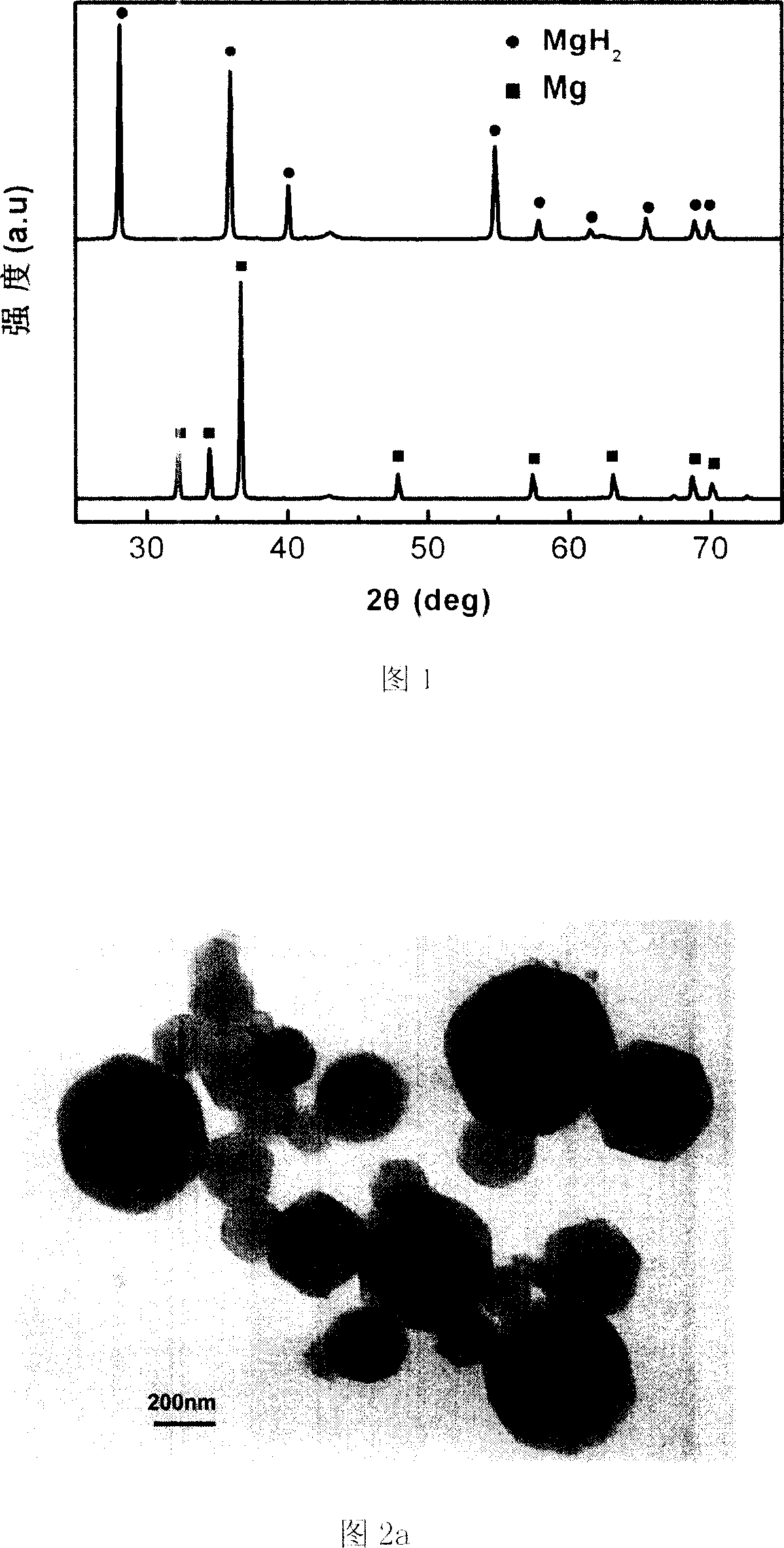
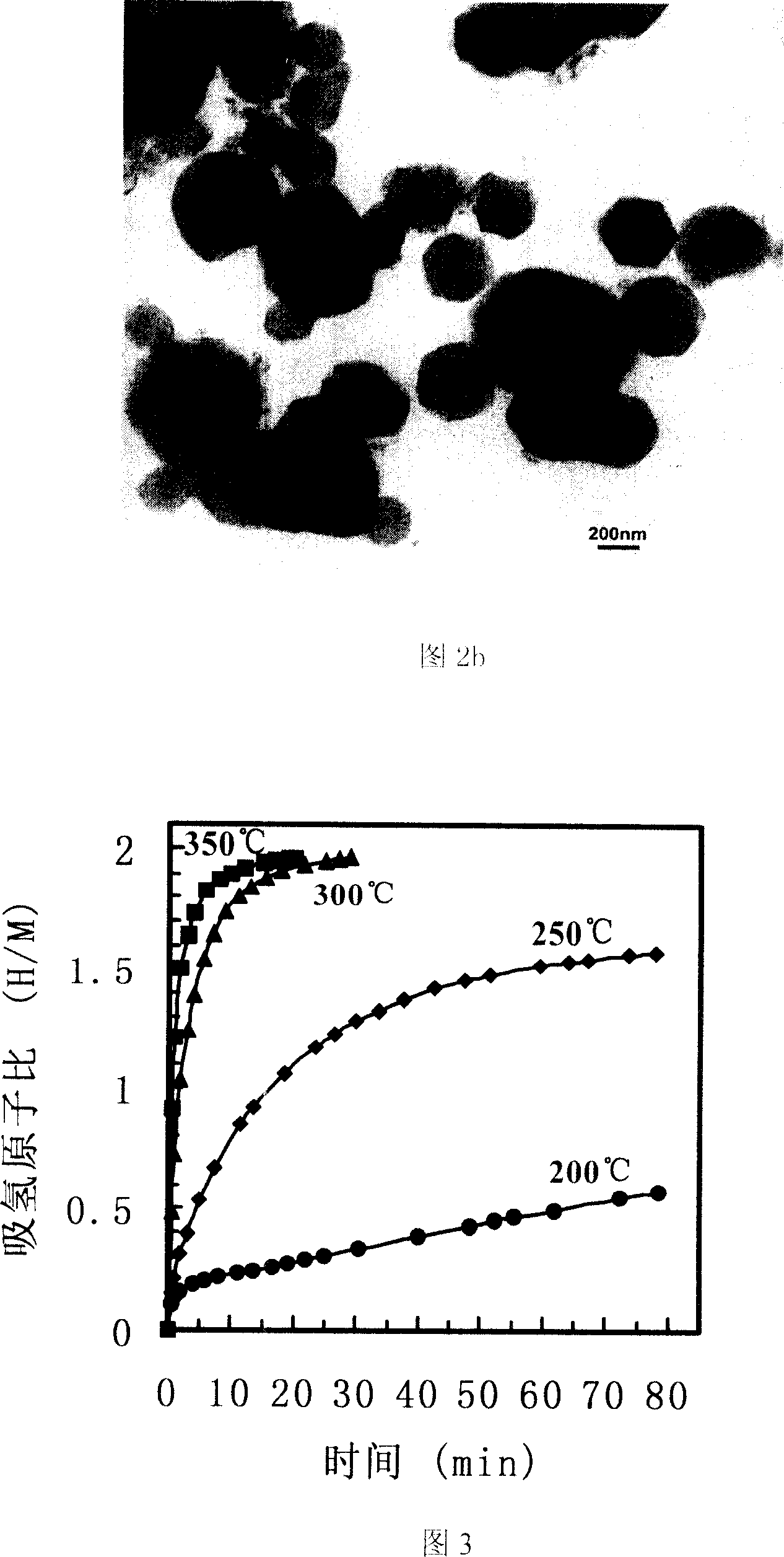
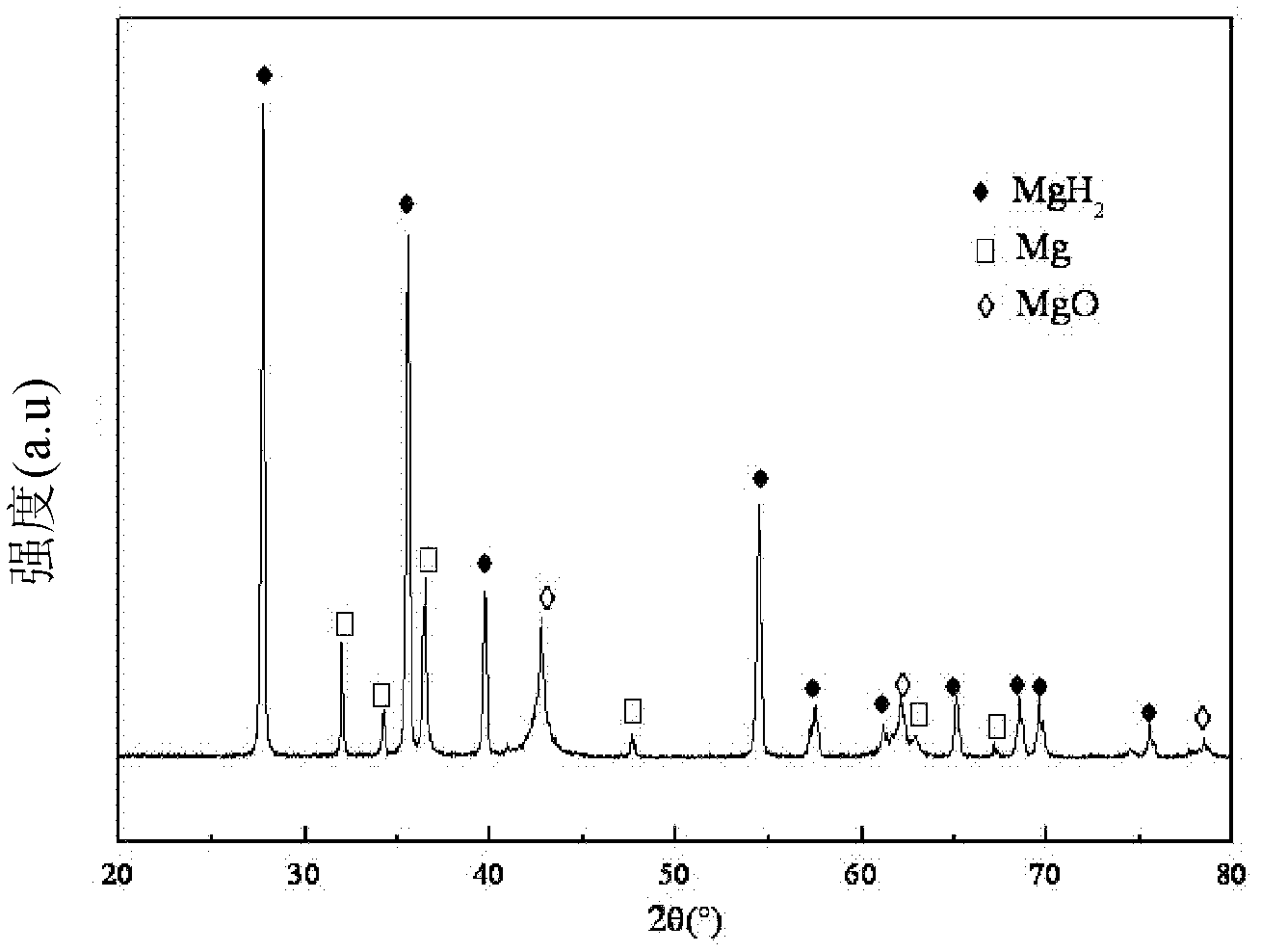
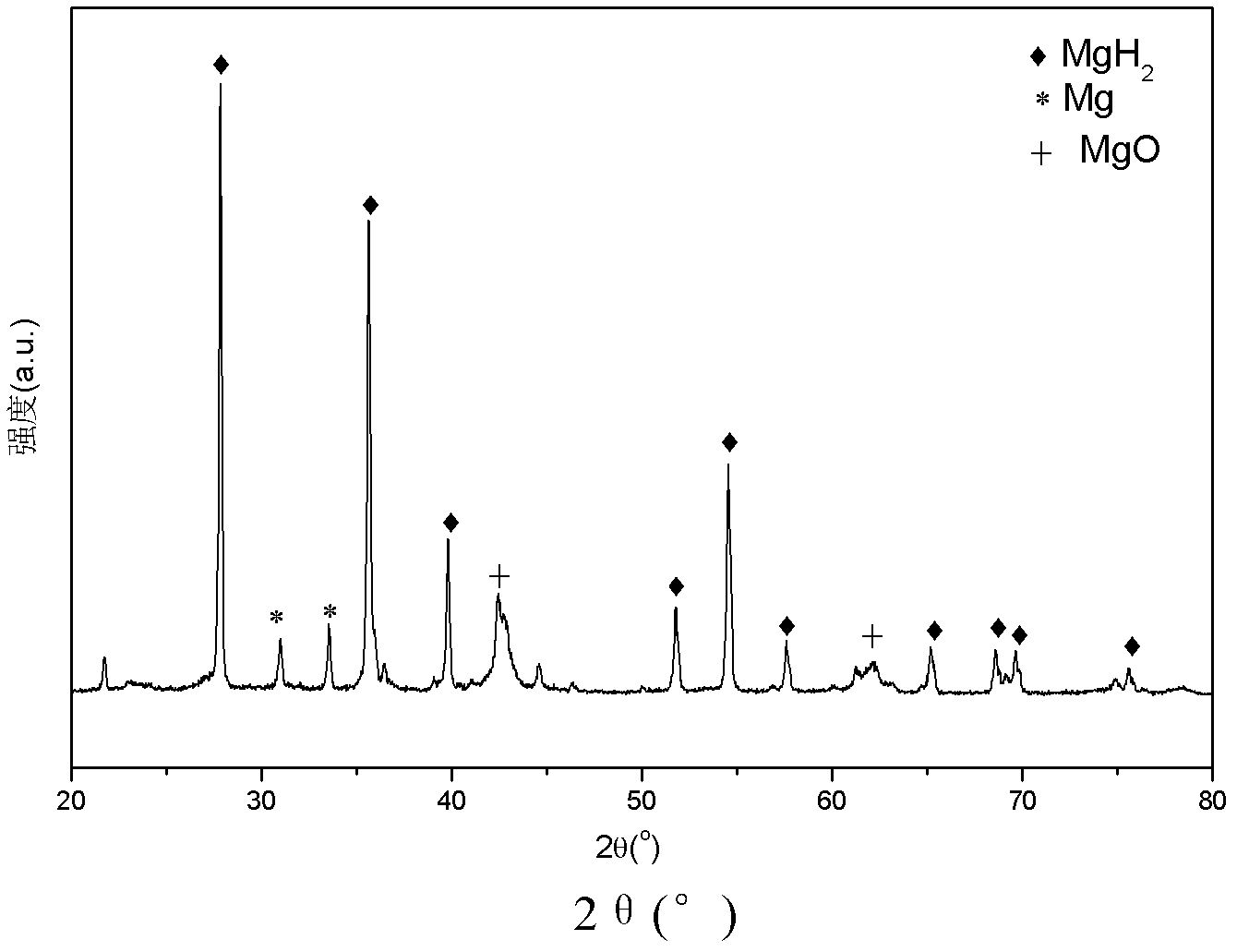
Method for preparing magnesium based hydrogen storage material
InactiveCN101003360AHigh purityHigh hydrogen storage capacityAlkali/alkaline-earth/beryllium/magnesium hydridesHydrogen pressureMechanical property
This invention relates to a method for preparing Mg-based hydrogen-storage material, especially a method for rapidly preparing hydride. The method comprises: (1) loading pure Mg powder into a reactor, and partially hydriding Mg at 473-723 K and 1-6 MPa hydrogen pressure for 2-12 h; (2) ball-milling the obtained MgH2 for 30 min-12 h; (3) re-loading into the reactor, performing desorption / adsorption of hydrogen to completely convert the material into MgH2 in a short time. The method has such advantages as simple process, no need for any additive, high sample purity, and low hydride cost. The prepared MgH2 has excellent hydrogen-storage performance and good mechanical properties. The hydrogen discharge amount is 2.3-4.5 wt. %. After second hydriding, the purity of MgH2 is higher than 95%. This invention provides a new route to prepare practible Mg-based hydrogen-storage material.
Owner:TAIYUAN UNIV OF TECH
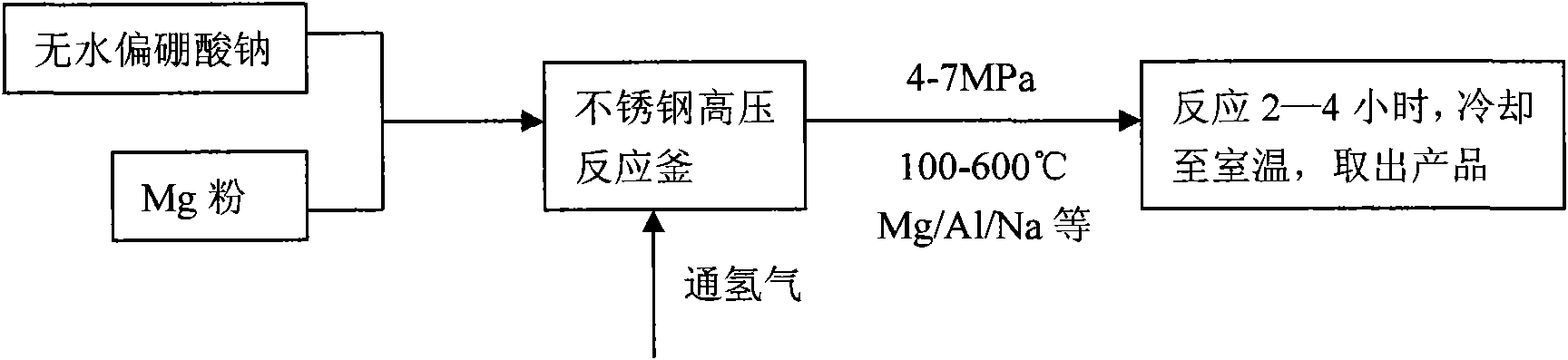
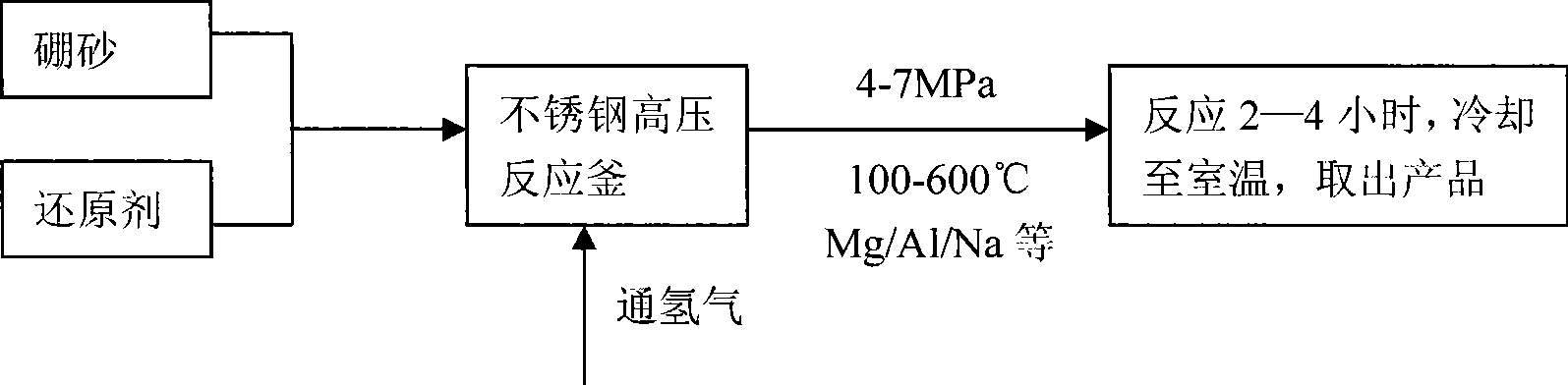
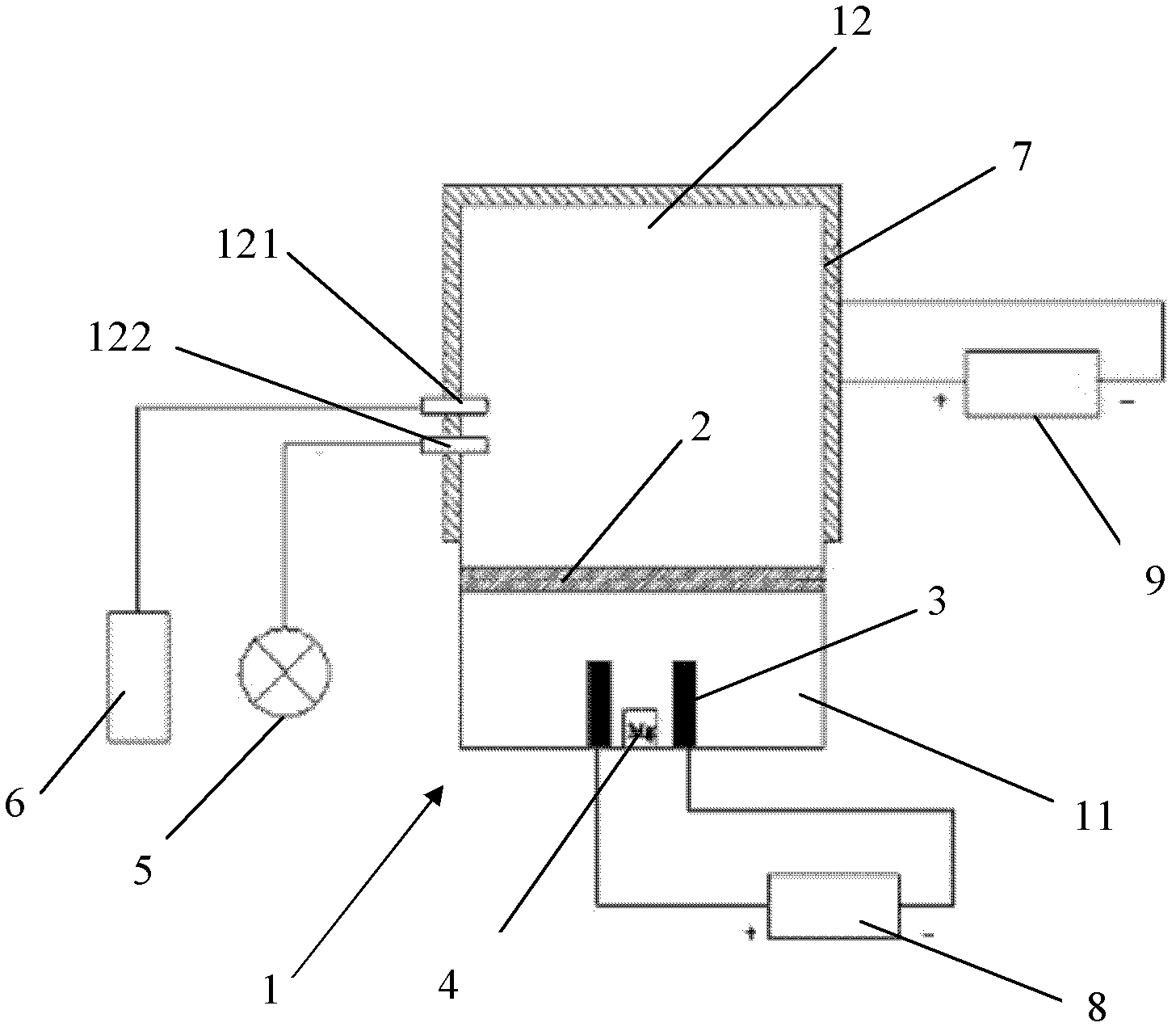
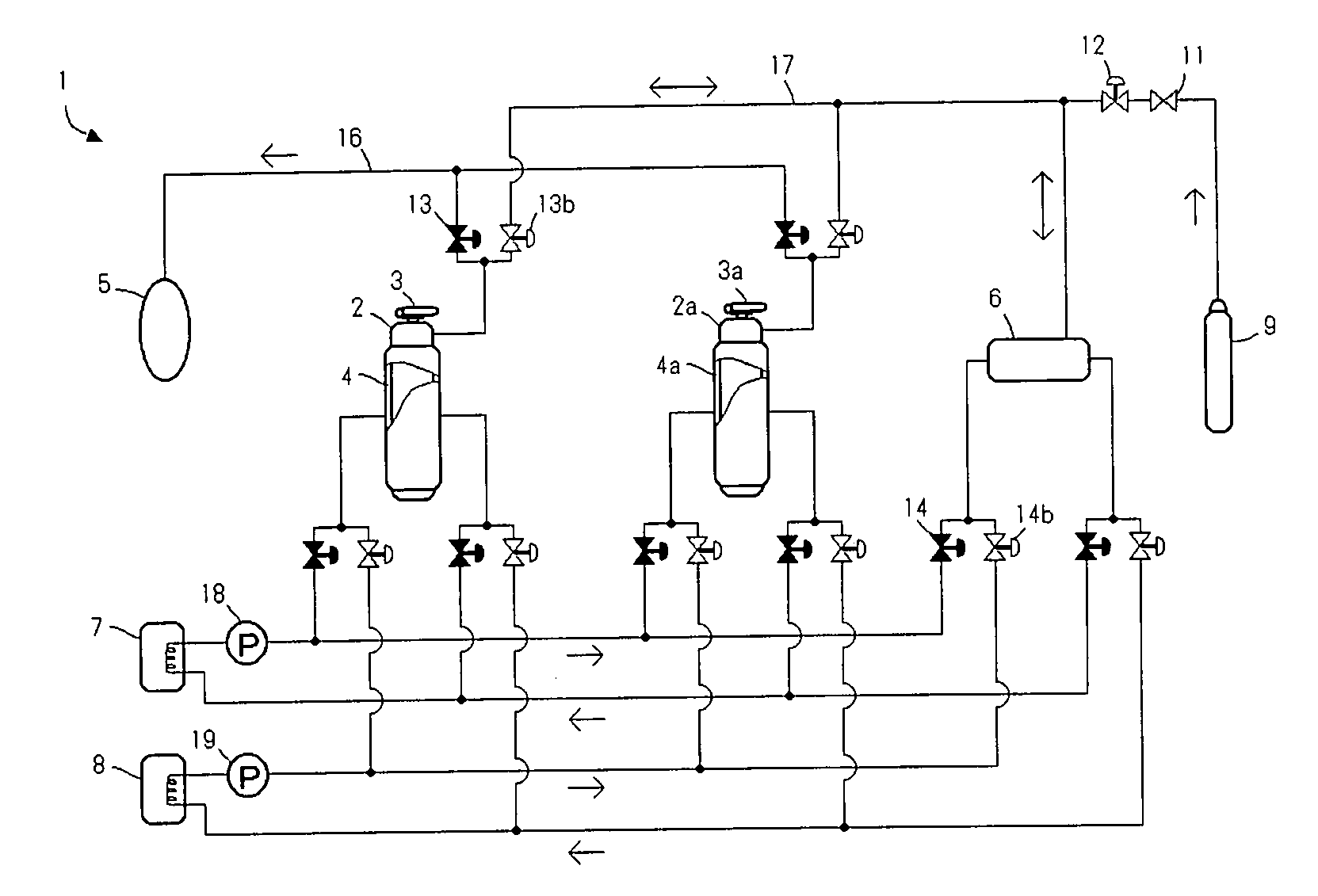
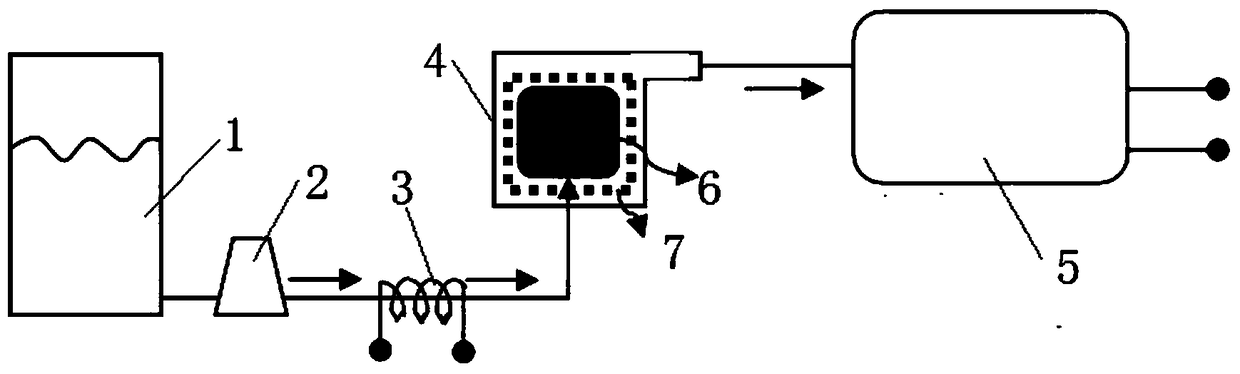
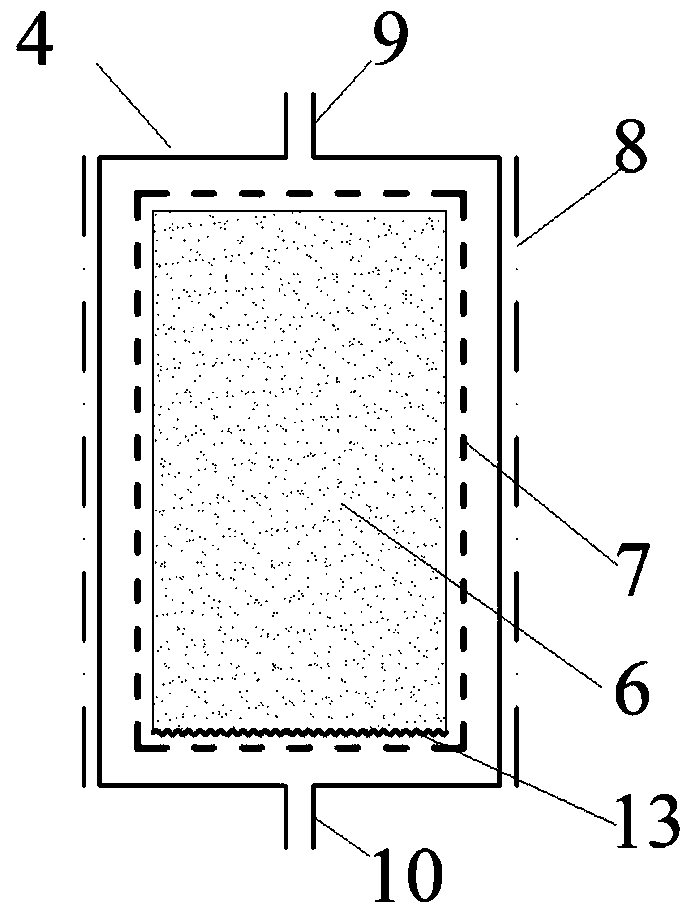
Method and device for preparing magnesium hydride
ActiveCN102583244AHigh puritySimple processAlkali/alkaline-earth/beryllium/magnesium hydridesHydrogen pressureEngineering
The invention relates to a method for preparing magnesium hydride and also relates to a device for preparing the magnesium hydride. The device for preparing the magnesium hydride comprises a reaction kettle, an inserted plate valve, a first heating coil, a second heating coil, a first gas inlet, a gas inlet and outlet port and a gas outlet, wherein the inserted plate valve is arranged in the reaction kettle and is positioned at the middle part of the reaction kettle to divide the reaction kettle into an upper part and a lower part; the first heating coil is arranged at the lower part of the reaction kettle; the second heating coil is arranged at the upper part of the reaction kettle; the first gas inlet is arranged on the side wall of the upper part of the reaction kettle; the gas inlet and outlet port is arranged on the side wall of the upper part of the reaction kettle; and the gas outlet is arranged on the side wall of the upper part of the reaction kettle. In the method for preparing the magnesium hydride, the device for preparing the magnesium hydride is adopted. The device disclosed by the invention solves the technical problem that a high temperature and a high hydrogen pressure are required for preparing the magnesium hydride currently.
Owner:浙江镁源动力科技有限公司
Oxygen direct-injection pure-hydrogen combustion engine and power system thereof
ActiveCN108443010AIncrease diversityIncrease flexibilityCellsHydrogenVapor–liquid separatorHigh pressure oxygen
The invention relates to an oxygen direct-injection pure-hydrogen combustion engine and a power system thereof. The oxygen direct-injection pure-hydrogen combustion engine comprises a hydrogen fuel engine body, a magnesium hydride storage tank, a pressure swing adsorption oxygenerator, a three-way catalyst, a tail gas waste heat utilization unit and a hydrogen purification unit. The magnesium hydride storage tank is connected to a hydrogen opening of the hydrogen fuel engine body through a low-pressure hydrogen buffering tank, and the pressure swing adsorption oxygenerator is connected to an oxygen nozzle of the hydrogen fuel engine body through a high-pressure oxygen buffering tank. Exhausting holes of the hydrogen fuel engine body are connected to the three-way catalyst through a tail gas expander or a turbocharging unit, and an outlet of the three-way catalyst is connected to a gas-liquid separator through a kelaipu unit / combined type kelaipu unit. A gas outlet of the gas-liquid separator is connected to a steam inlet of the magnesium hydride storage tank through the hydrogen purification unit, and a water outlet of the gas-liquid separator is connected to a cooling water tank.The oxygen direct-injection pure-hydrogen combustion engine is combined with the kelaipu unit / combined type kelaipu unit through the hydrogen fuel engine body, tail gas waste heat of the engine body is fully utilized, and the heat efficiency of the engine body is improved.
Owner:SHANGHAI KELAIPU ENERGY TECH CO LTD
Method of rapidly carrying out hydrogenation of hydrogen storage material
InactiveCN1478055AAlkali/alkaline-earth/beryllium/magnesium hydridesReversible hydrogen uptakeHydrogen pressureRoom temperature
Disclosed is a method for rapidly carrying out a hydrogenation of a material capable of absorbing hydrogen. It was discovered that when a powder of a material capable of absorbing hydrogen is ground under a hydrogen pressure, not at room temperature but at a higher temperature (about 300 DEG C. in the case of magnesium) and in the presence of a hydrogenation activator such as graphite and optionally a catalyst, it is possible to transform completely the powder of this material into a hydride. Such a transformation is achieved in a period of time less than 1 hour whereas the known methods call for periods of time as much as 10 times longer. This is an unexpected result which gives rise to a considerable reduction in the cost of manufacture of an hydride, particularly MgH2.
Owner:HYDRO QUEBEC CORP
Hydrogen energy systems
ActiveUS8651268B2Safe storageControl releaseReversible hydrogen uptakeReactant parameters controlHydrogenIn vehicle
Hydrogen energy systems for obtaining hydrogen gas from a solid storage medium using controlled laser beams. Also disclosed are systems for charging / recharging magnesium with hydrogen to obtain magnesium hydride. Other relatively safe systems assisting storage, transport and use (as in vehicles) of such solid storage mediums are disclosed.
Owner:SMITH PAUL H JR
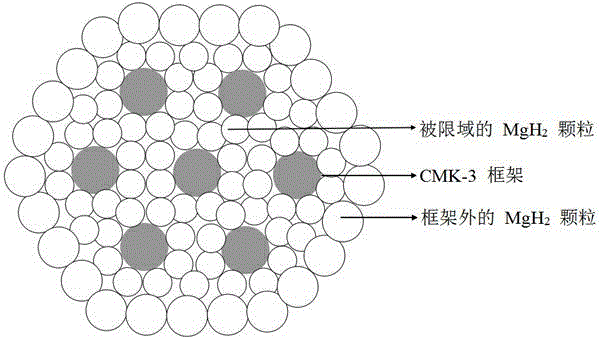
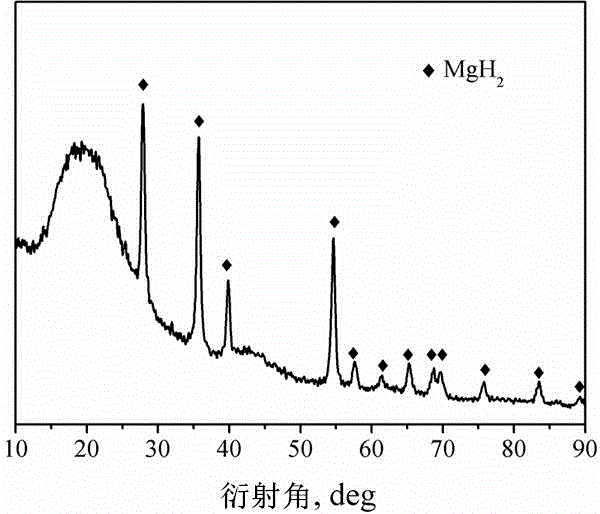
Method for preparing nanometer limited range magnesium-based hydrogen storage material
InactiveCN104649229AImprove mechanical propertiesLow costAlkali/alkaline-earth/beryllium/magnesium hydridesDispersityNew energy
The invention discloses a method for preparing a nanometer limited range magnesium-based hydrogen storage material. The nanometer limited range method belongs to the technical field of new energy materials. The method is characterized in that the hydrogen storage material is formed by loading magnesium hydride (MgH2) in nanopores of a mesoporous framework material. The method comprises the following steps: dipping dibutyl magnesium (MgBu2) and a mesoporous framework material, replacing MgBu2 with MgH2 loaded inside and outside the nanopores of the mesoporous framework material in a high-pressure reactor under high temperature and high pressure, washing MgH2 loaded outside the pores away by using pentane, drying, thereby obtaining the material. The nanometer limited range magnesium-based hydrogen storage material prepared by the method can release hydrogen at room temperature and has excellent properties in hydrogen absorption and desorption kinetics and hydrogen desorption thermodynamics. The method disclosed by the invention is easy to operate and high in synthesis speed and the prepared material is high in dispersity; therefore, the method has ideal application prospects.
Owner:SHANGHAI UNIV
Magnesium hydride based composite powder
InactiveCN102060266AAccelerate the rate of hydrogenationSmall particle sizeHydrogen productionWater qualityCopper oxide
The invention relates to a magnesium hydride based composite powder. The magnesium hydride based composite powder is prepared by taking nano Mg powder as a base material. The quality percentage contents of the metal particles and the metal oxide particles in the mixture are as follows: Mg powder accounts for 95-99%, the metal particles account for 0.5%-4.0% and the metal oxide particles account for 0.5%-4.0%. The metal particles are one or more of manganese, iron, cobalt, nickel and copper. The metal oxide particles are one or more of manganese oxide, iron oxide, cobalt oxide, nickel oxide and copper oxide. Nano crystal hydrogenated magnesium composite powder is obtained by filling the mixture through hydrogen filling ball-milling in a ball grinder. The invention has the advantages of reasonable composition, simple preparation process, low cost, hydrogen storage performance and remarkably dynamics property, is used for hydrolyzing for producing hydrogen, and has better water quality adaptability and temperature adaptability; and a hydrolysis product is safe and non-toxic so as to be environmental-friendly.
Owner:张文丛
Hydrogen Energy Systems
ActiveUS20100163434A1Increase heightSafe storageReactant parameters controlReversible hydrogen uptakeHydrogenIn vehicle
Hydrogen energy systems for obtaining hydrogen gas from a solid storage medium using controlled laser beams. Also disclosed are systems for charging / recharging magnesium with hydrogen to obtain magnesium hydride. Other relatively safe systems assisting storage, transport and use (as in vehicles) of such solid storage mediums are disclosed.
Owner:SMITH PAUL H JR
Functional product, treatment device of functional substance, applied device of functional product and mounting method of functional product
InactiveUS20090035623A1Undesirable effectReactant parameters controlRadiation applicationsAutomatic controlSolvent
The present invention relates to a production and a usage of a functional substance of a fine powder of nanometer size, and to an improved treatment device of the functional substance and an improved applied device of a functional product, further, to a weight reduction of a device using the functional substance and a production cost reduction.The functional product uses a functional product means in which a fine functional powder of nanometer size or a fine functional powder of nanometer size packed together using a coating material is made into a solid or granular membrane. A treatment device of a functional substance comprises: single or plural of treatment container means which is a pressure-resistant container provided with a flange, a jacket and a temperature control section; a hydrogen filling means having a deaerating device and a hydrogen storage and release device which are mounted on said pressure-resistance container; a heating and cooling device having a heating device and a cooling device which are mounted on each of said pressure-resistant container and said hydrogen storage and release device; and an electric control means for automatically controlling said treatment container, said hydrogen filling means and said heating and cooling means. A storage device for a functional product comprises a energy conversion and storage means for sealing and storing a functional product, which has been subjected to a reduction and a hydrogenation of a functional substance using natural or regenerable energy, in a waterproof container or bag. A molded product, a painting material, a coating material and a grouting material using said functional product comprises a catalytic means in which a material, a binder and a functional catalytic product are mixed and dispersed. A hydrogen solvent using said functional product comprises a hydrogen solution means in which a functional product of a hydrogenated functional substance is mixed with a granular, solid or viscous medicine, food or adhesive membrane material, or is filled in a container. A hydrogen utilization device using said functional product comprises: a hydrogen discharge means comprising a hydrogen generating container on which a functional product consisting of a functional hydrogenated substance is mounted; a hydrogen storage and release means comprising a hydrogen storage container using a hydrogen storage substance on which a heating device is attached; a hydrogen generating means for reacting said functional product of said hydrogen discharge means with liquid water to form a metal hydride and to generate a hydrogen gas from a hydrolysis; a row material water supply means for supplying the hydrogen gas generated by said hydrogen generating means to a hydrogen utilization body and applying water being compounded with oxygen to said hydrogen generating means; and an electric control means containing a detection system; wherein these means are constructed in a unit. A gas sensor using said functional product comprises: a composite element means comprising a gas reactant on which a measuring junction of a thermoelectric couple and a functional product are mounted; a detachable means for storing said composite element means in a detachable container; and an electric control means comprising an electric control unit consisting of a power source for controlling said composite element means, a Thomson effect control system and a Seebeck effect control system and the like. A secondary battery using said functional product comprises: an electrode means formed by using a functional product of an active substance and a low-temperature plastic coating material; and an unifying means bonding power generating elements comprising a negative electrode, a positive electrode and a separation membrane of said electrode means and covering the elements with an insulation membrane. A fuel cell or reversible fuel cell in which said functional product is used in a MEA (Membrane Electrode Assembly) plate comprises: a MEA (Membrane Electrode Assembly) plate means in which a MEA (Membrane Electrode Assembly) is formed on one side of a plate formed with a corrugated portion; a stacking means stacking a single body of said MEA (Membrane Electrode Assembly) plate, a MEA (Membrane Electrode Assembly) cassette in which two of said MEA (Membrane Electrode Assembly) plates are stacked, or the MEA (Membrane Electrode assembly) cassettes; and a sealing means covering said single body of MEA (Membrane Electrode Assembly) plate or said MEA (Membrane Electrode Assembly) cassette with a coating material, or, bonding the periphery of said stacked MEA (Membrane Electrode Assembly) cassettes, sealing and separating an inside of a positive electrode or a negative electrode and providing nozzles for a fluid of two passages. Accordingly, the present invention provides the following advantages. A fine powder of a metal hydride and a metal powder made by a reduction of a metal compound can be produced at low cost. Even when a fine powder of an activated functional substance is exposed in air for a long period, it is safe and firing and poisoning do not occur. This solves a conventional problem. And, the product can be easily mounted various devices with low cost. In addition, by producing magnesium hydride using natural or renewable energy, it makes possible to convert the energy into a high-density safe substance and to store and transport. Furthermore, a large quantity of hydrogen can be supplied to a hydrogen utilization body. And, a long-lived gas sensor, secondary cell and the like can be obtained.
Owner:TECH BANK CO LTD
Magnesium base hydride composite system for hydrolysis hydrogen production and preparation method and application thereof
InactiveCN101811667ASimple processEase of industrial mass productionAlkali/alkaline-earth/beryllium/magnesium hydridesHydrogen productionCombustionGraphite
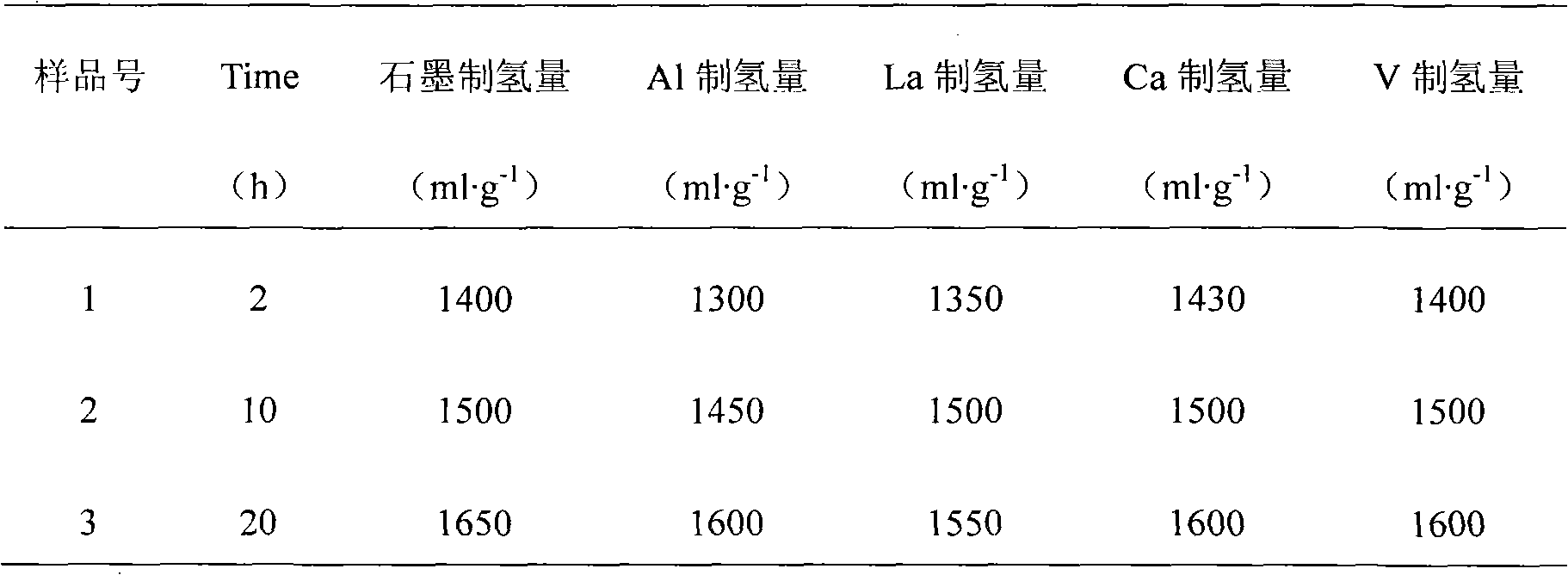
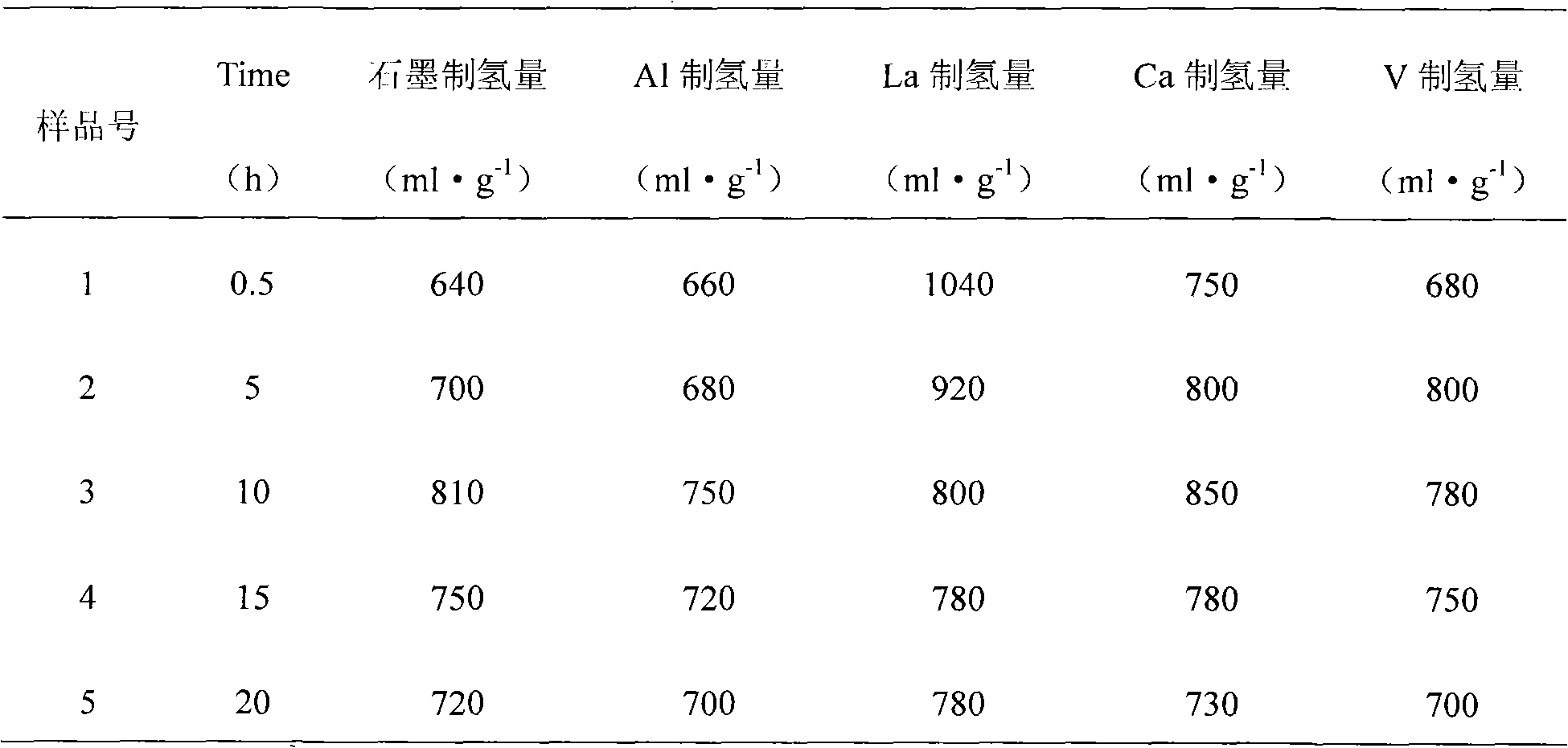
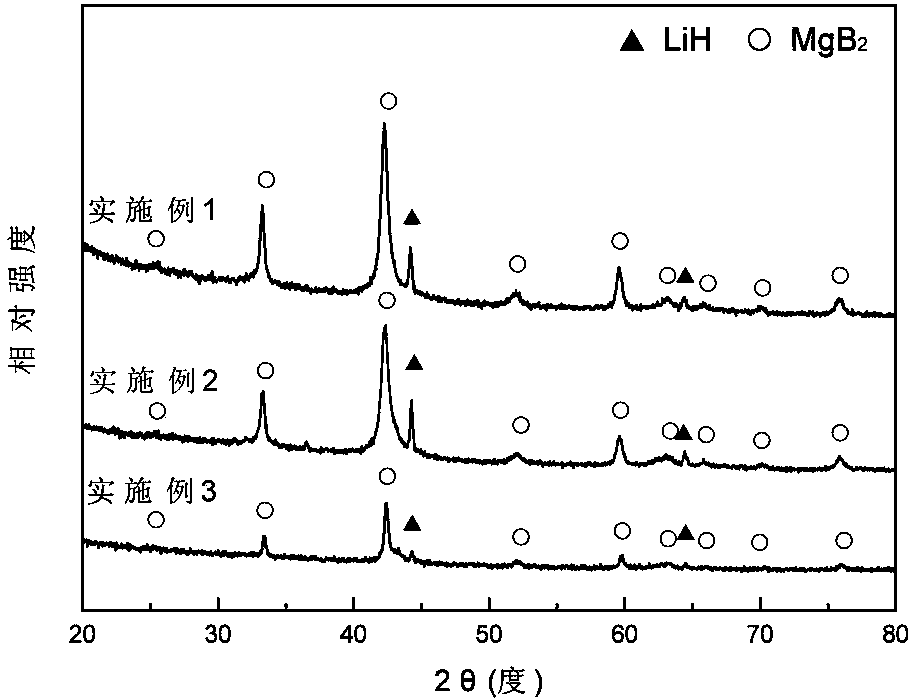
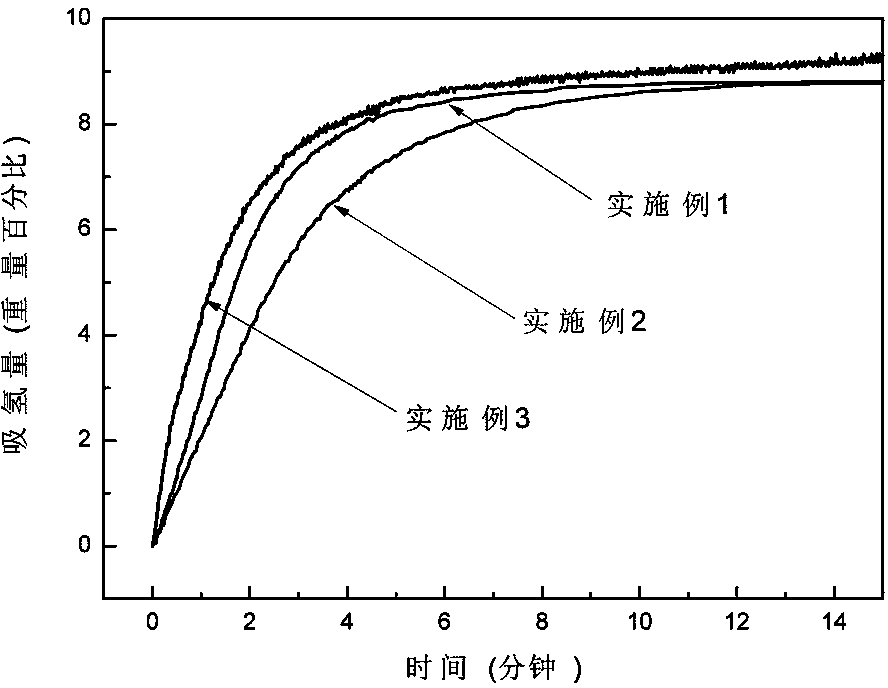
The invention discloses a magnesium base hydride composite system for hydrolysis hydrogen production and a preparation method and application thereof. The composite system is prepared by a method comprising the following steps of: taking magnesium powder and nickel powder in a mole ratio of 30:1-49:1; performing hydrogenation combustion synthesis under the action of a catalyst and an organic dispersant which account for 3 to 10 percent of the total weight of the mixture of the magnesium powder and the nickel powder; and performing strong mechanical ball milling. The magnesium hydride in the prepared magnesium base hydride composite system for the hydrolysis hydrogen production accounts for 95 to 98 percent of the weight of hydride, and the magnesium nickel hydride accounts for 2 to 5 percent; the catalyst is one of graphite, B, Al, La, Ca, V, Ce and Nb; the magnesium hydride is MgH2; and the magnesium nickel hydride is a mixture of Mg2NiH4 and Mg2NiH0.3 in any ratio. The theoretical hydrogen production amount of the composite system is up to 1,600 ml / g, and a preparation process of the magnesium base hydride composite system is time-saving, energy-saving and simple and is easy for industrial production.
Owner:NANJING UNIV OF TECH
Preparation method of metal oxide composite magnesium hydride hydrogen storage material
PendingCN110980635AAccelerate the rate of hydrogenationSmall particle sizeHydrogenOxide compositeBall mill
The invention belongs to the technical field of hydrogen storage materials, and particularly relates to a preparation method of a metal oxide composite magnesium hydride hydrogen storage material. Themethod comprises the steps that magnesium hydride serves as a base material, metal oxide particles are mixed, the mass percentage content of metal oxide in the mixture is 1%-8%, and the mixture is subjected to hydrogen filling ball milling in a ball mill to obtain the metal oxide composite magnesium hydride hydrogen storage material. By adding the nanoscale metal oxide particles, the hydrogenation speed of the magnesium-based composite powder in the hydrogen charging ball milling process can be increased, and reduction of the particle size of magnesium hydride is facilitated; the nanocrystalline MgH2 is coated with the metal oxide to form a core-shell structure with an excellent synergistic effect, the core-shell structure has a good catalytic effect and remarkable dynamic performance, the hydrogen storage reaction speed can be improved, microcell heat release can be controlled, agglomeration of a hydrogen storage reactant Mg (OH) 2 can be effectively eliminated through the metal oxide, and the hydrogen storage efficiency is improved. Therefore, the purpose of sufficient hydrolysis reaction is achieved.
Owner:世能氢电科技有限公司 +1
Method for synthesizing lithium borohydride
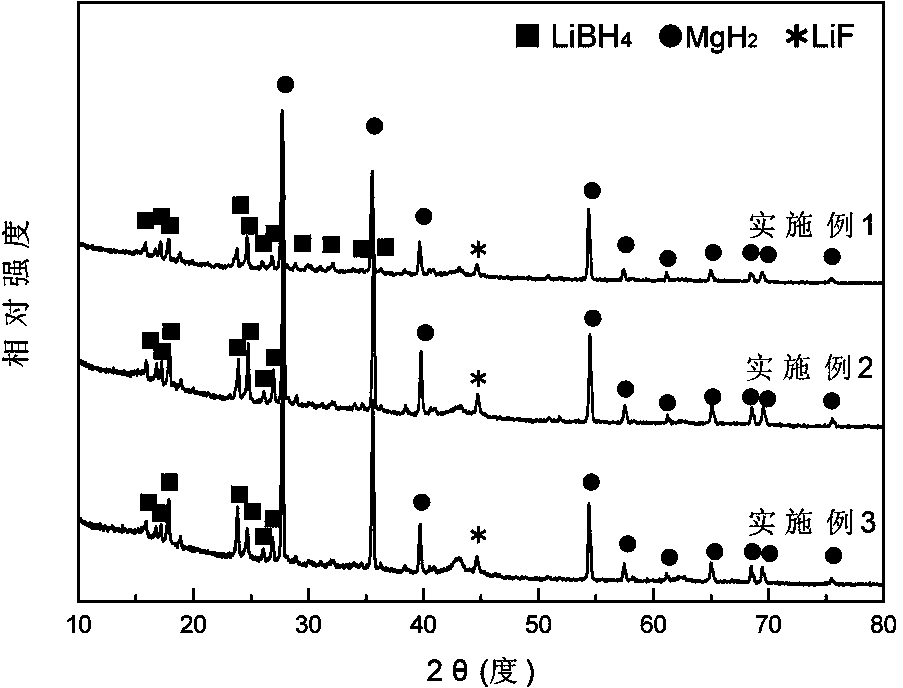
ActiveCN103922285ASimple reaction conditionsShort reaction timeMonoborane/diborane hydridesMagnesium diborideHydrogen absorption
The invention discloses a method for synthesizing lithium borohydride. The method aims at solving the problems that an existing method for preparing lithium borohydride is complex in technology, strict in synthesis condition and the like. The method comprises the following steps that in a protective atmosphere, after lithium hydride, magnesium diboride and a catalyst are mixed according to a ratio, ball-milling treatment is conducted, and a product is obtained after ball-milling treatment; a hydrogen absorption reaction is conducted on the product which is obtained after ball-milling treatment, so that a mixture of the lithium borohydride and magnesium hydride is obtained and is recorded as a reaction product; the magnesium hydride and the lithium borohydride in the reaction product are separated, so that the lithium borohydride is obtained. The method overcomes the shortages that a traditional method for preparing the lithium borohydride is complex in technology and strict in synthesis condition and the purity of the product is low and the like. The method is simple, gentle in reaction condition and short in reaction time. Synthesized LiBH4 is high in yield and purity. Meanwhile, through the method, the production period of the LiBH4 can be shortened, the production cost is reduced, the lithium borohydride can be produced in a large scale industrially, and application of the lithium borohydride is facilitated.
Owner:SICHUAN INST OF MATERIALS & TECH
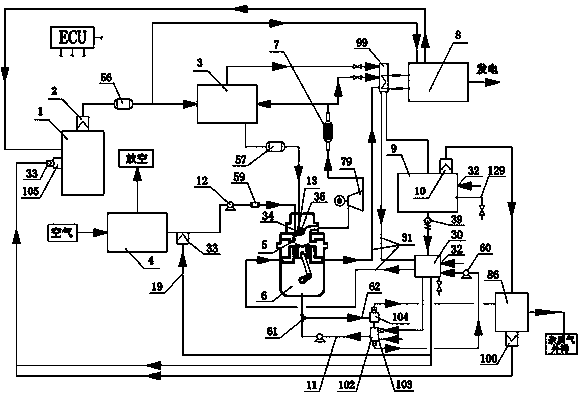
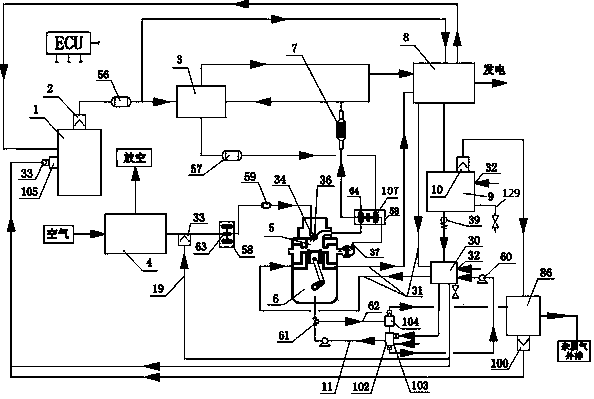
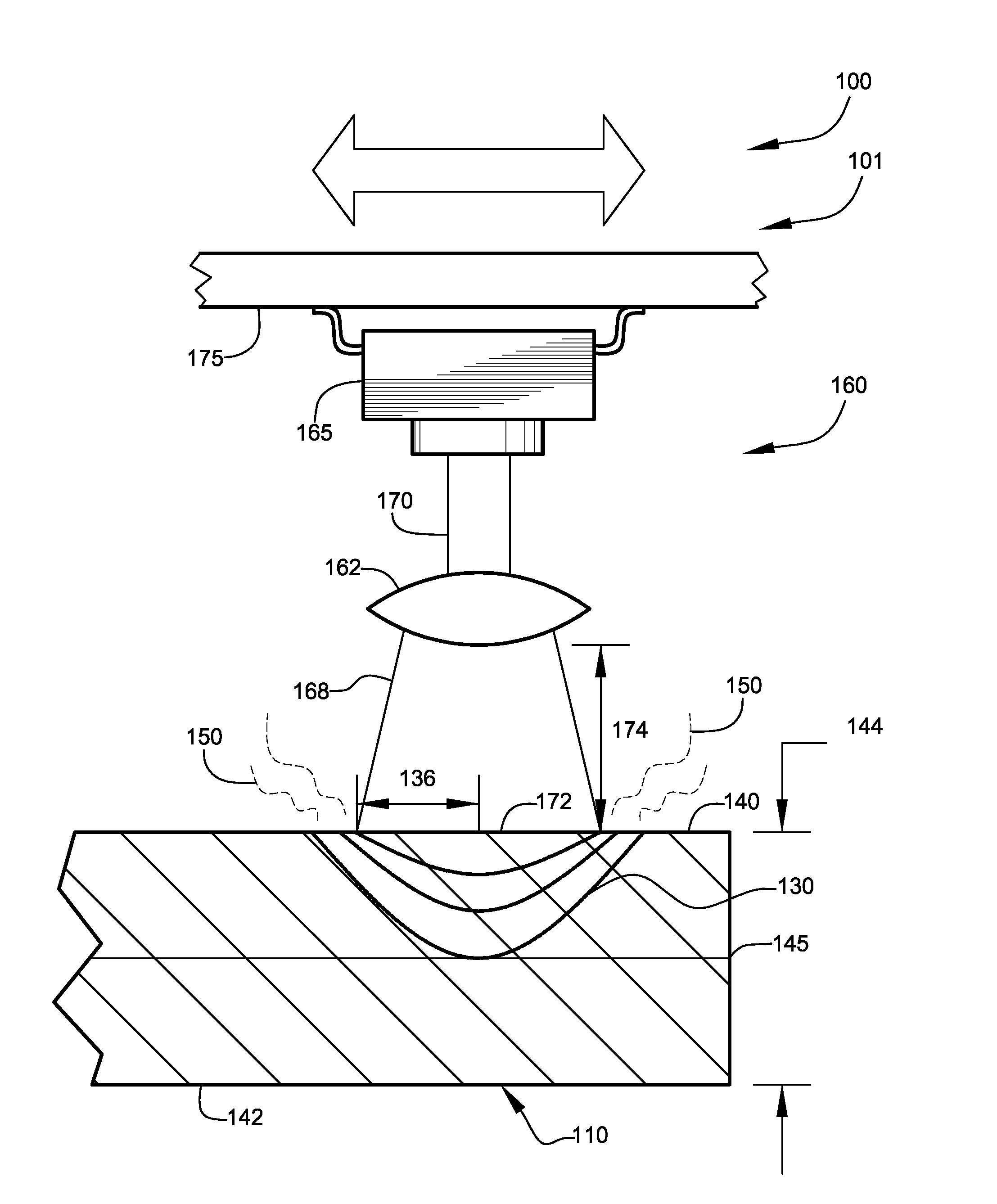
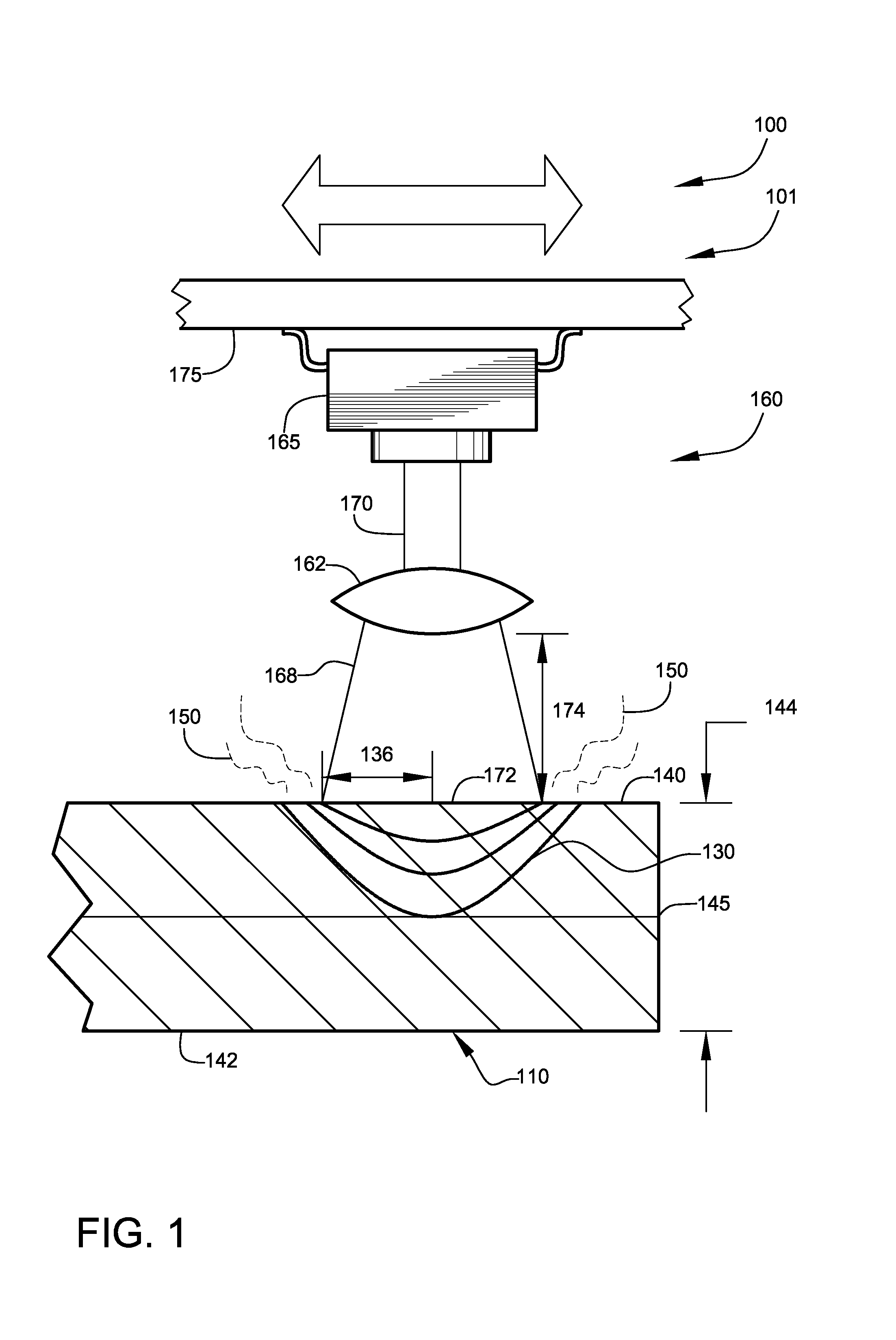
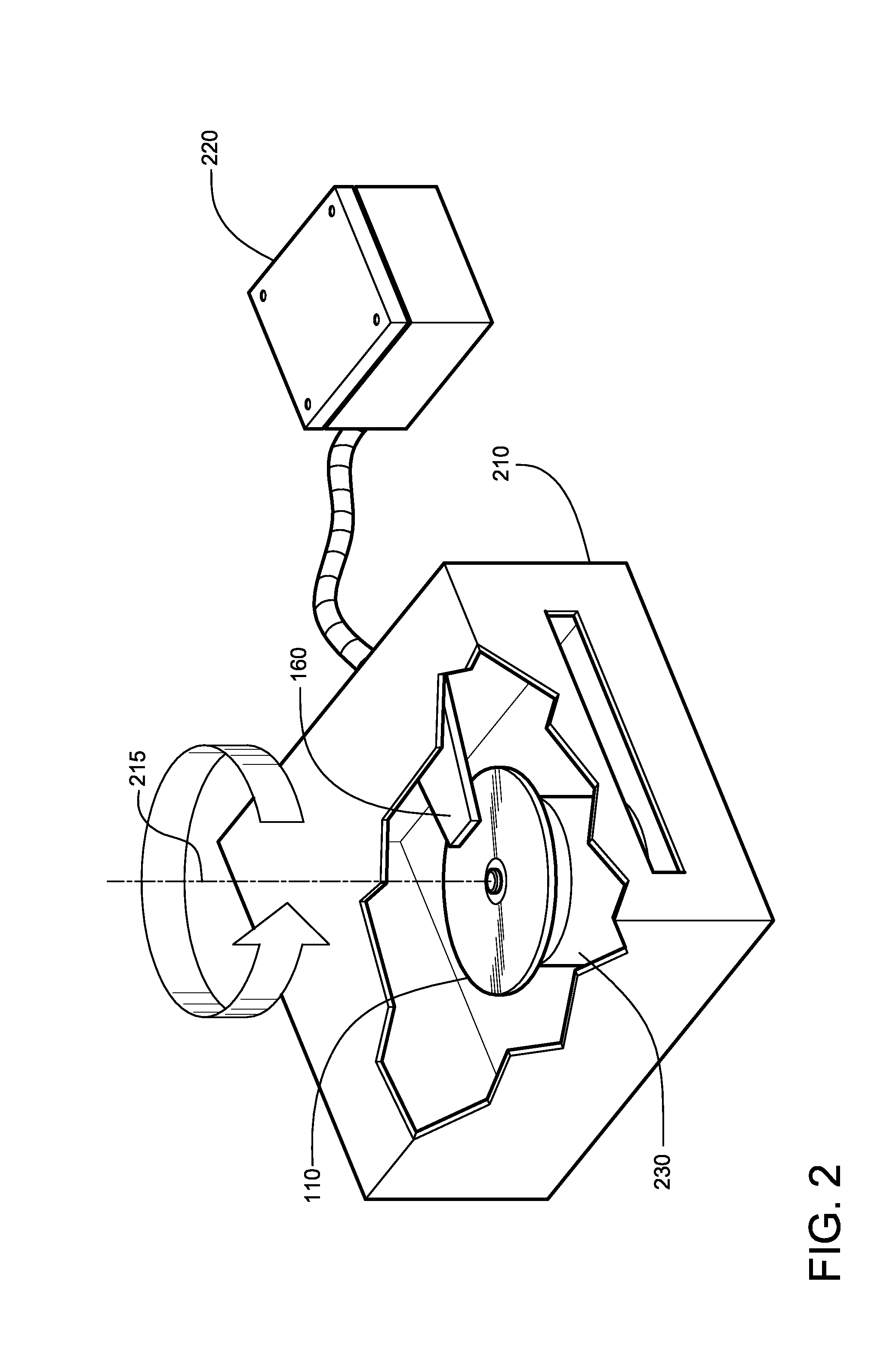
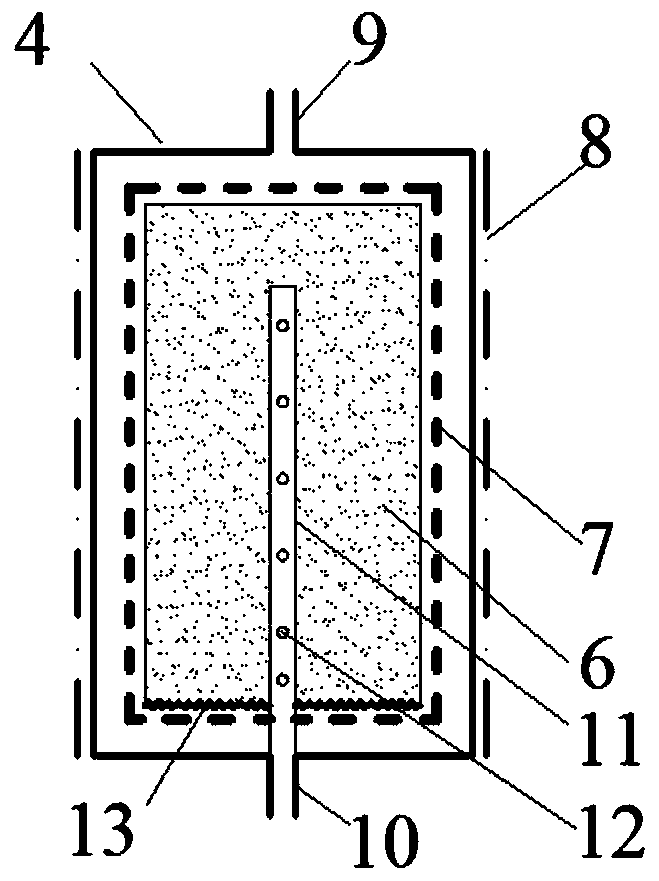
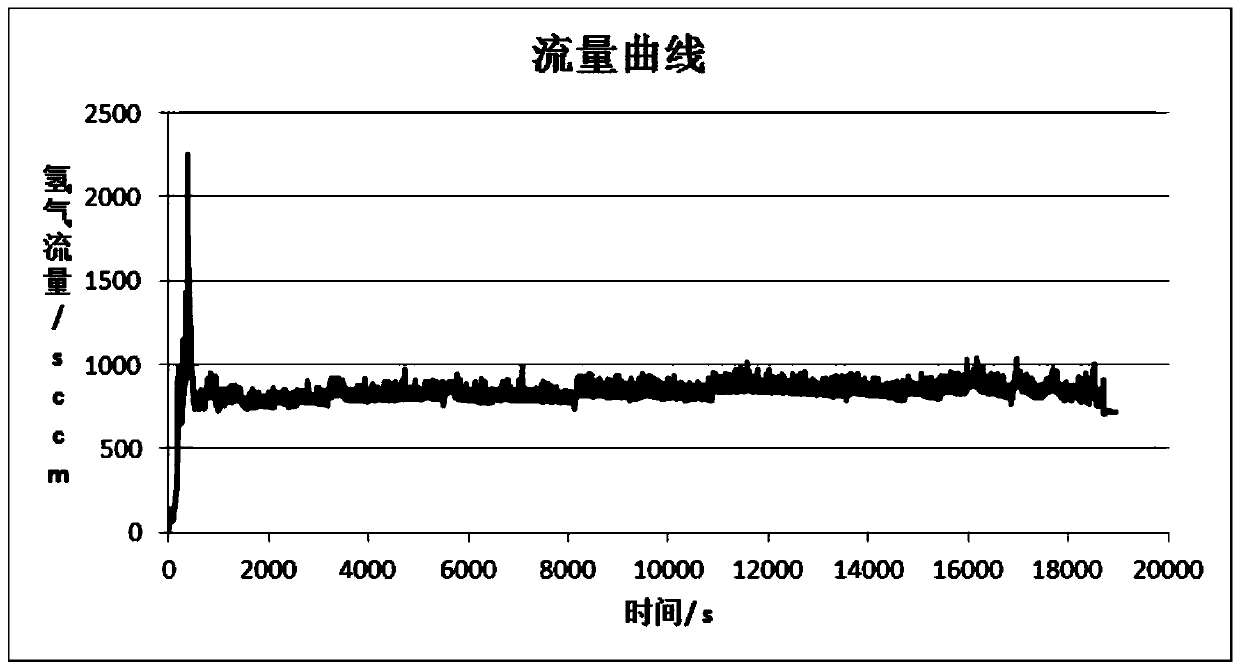
Hydrogen production method and device with magnesium hydride as hydrogen storage material
The invention relates to the technical field of hydrogen production, and in particular, relates to a hydrogen production device with magnesium hydride as a hydrogen storage material, wherein the hydrogen production device includes a reaction chamber; a massive magnesium hydride filler layer is arranged in the reaction chamber. Provided is a hydrogen production method with magnesium hydride as thehydrogen storage material; massive magnesium hydride is prepared by mixing and pressing MgH2 and other chemical substances (M), the MgH2 / M ratio is 10 wt.%-80 wt.%, and the massive magnesium hydride is shaped as particles or cakes, wherein the particles are spheres or irregular spheres with the diameter of 1-50 mm, and the cakes are round cakes or cakes with other shapes with the bottom diameter or side length of 1-100 mm and the height of 1-50 mm. The massive magnesium hydride is stacked and filled in the reaction chamber, the void between the particles becomes larger and the energy density of the filler increases so as to be conducive to full contact reaction of water vapor to all parts of the filler to produce hydrogen with high efficiency. At the same time, hydrogen gas escapes from the filler more smoothly to prevent the sudden increase of the pressure in the reaction chamber. The start-up time of reaction is shortened by adding a reaction promoter, and the quality of hydrogen gasis improved by adding a gas purifier.
Owner:武汉市能智达科技有限公司
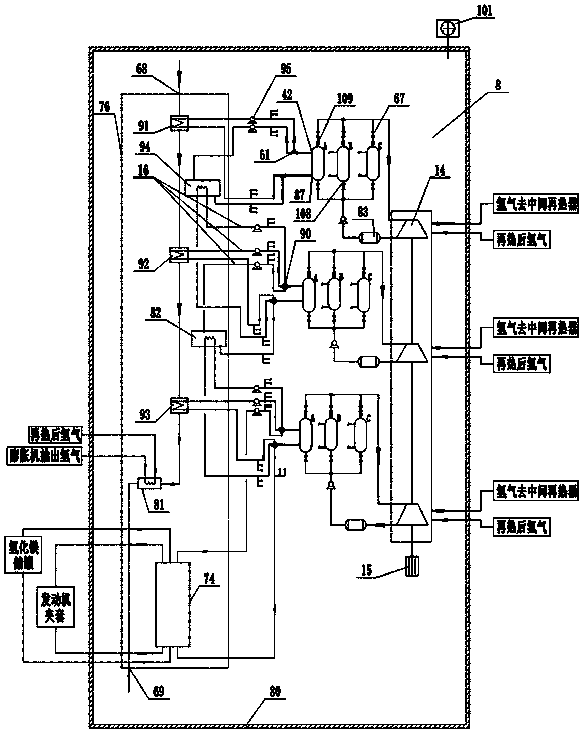
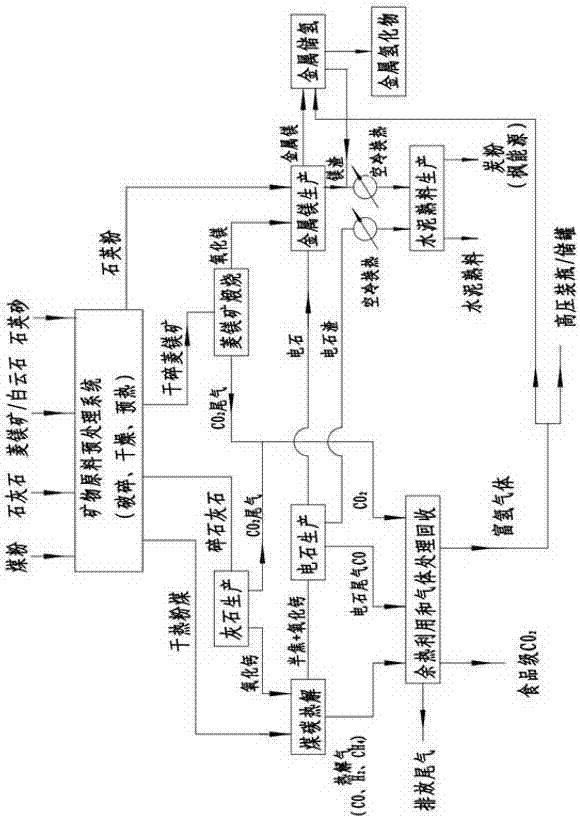
Rotary kiln and poly-generation system utilizing rotary kiln for producing metal hydride
InactiveCN107091569ARealize continuous productionNo emissionsFurnace componentsAlkali/alkaline-earth/beryllium/magnesium hydridesHigh concentrationBrick
The invention discloses a rotary kiln and a poly-generation system utilizing the rotary kiln for producing metal hydride. The poly-generation system comprises a lime production sub-system, a pulverized coal production sub-system, a calcium carbide production sub-system, a magnesium metal production sub-system, a waste heat utilizing and gas recycling sub-system, a metal hydrogen storage sub-system and a cement clinker production subsystem. Limestone, magnesite / dolomite, quartz sand and coal serve as raw materials, and the limestone is roasted into lime; in a lime kiln built through zirconia bricks, the lime and the coke powder are subjected to the high-temperature reaction so that liquid calcium carbide can be obtained; the magnesite / dolomite is roasted into magnesium oxide, and the calcium carbide, the magnesium oxide and the quartz sand are mixed to generate magnesium; high-concentration carbon dioxide, coal pyrolysis gas and calcium carbide tail gas obtained through decomposing of the limestone and the magnesite are recycled and processed, and hydrogen and food-level carbon dioxide are obtained; magnesium hydride is generated through union of hydrogen and magnesium; and carbide slag and magnesium slag are processed, and cement clinker and carbon powder are obtained. According to the poly-generation system, emission of solid waste is avoided, and carbon emission is greatly reduced.
Owner:SHIJIAZHUANG XINHUA IND FURNACE CO LTD
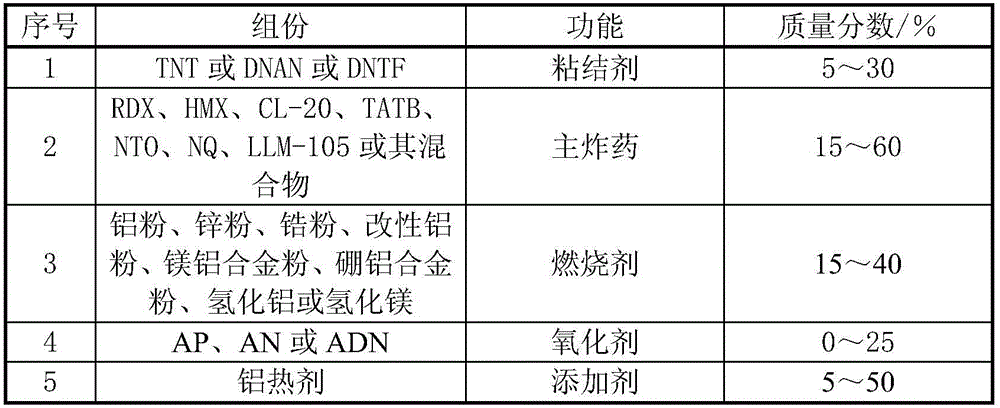
High-energy-density explosive mixture and preparation method thereof
The invention discloses a high-energy-density explosive mixture. A low-melting-point single-compound explosive is adopted and comprises, by mass, 5-30% of an adhesive, 15-60% of a main explosive, 15-40% of a combustion agent, 0-25% of an oxidizing agent and 5-50% of a high-density high-energy additive, wherein the adhesive is prepared from trinitrotoluene, 2,4-dimitroanisole and 3,4-dinitrofurazanofuroxan, the main explosive is prepared from RDX, HMX, CL-20, TATB, NTO, NQ, LLM-105 or the mixture thereof, the combustion agent is prepared from aluminum powder, zinc powder, zirconium powder, modified aluminum powder, magnalium powder, boron-aluminum alloy powder, aluminum hydride and magnesium hydride, the oxidizing agent is prepared from ammonium perchlorate, ammonium nitrate and ammonium dinitramide, and thermite is adopted as the high-density high-energy additive. The prepared explosive mixture has the high energy density, the density is larger than or equal to 2.0 g / cm<3>, and the heat of explosion in unit volume is larger than or equal to 16000 J / g.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
Application of magnesium hydride hydrogen production assistants, magnesium hydride hydrogen production mixed reagent and method for preparing hydrogen from magnesium hydride
PendingCN109824013AImprove production efficiencySimple processHydrogen productionDissolutionPollution
The invention belongs to the technical field of magnesium hydride hydrogen production, and particularly relates to application of magnesium hydride hydrogen production assistants, a magnesium hydridehydrogen production mixed reagent and a method for preparing hydrogen from magnesium hydride. According to the application of the magnesium hydride hydrogen production assistants, the magnesium hydride hydrogen production mixed reagent and the method for preparing the hydrogen from the magnesium hydride, one or the mixture of more of the magnesium hydride hydrogen production assistants is taken asan assistant for preparing the hydrogen from the magnesium hydride, wherein the magnesium hydride hydrogen production assistants are matter of which the dissolution enthalpy is less than zero; further, the magnesium hydride hydrogen production assistants are salt of which the dissolution enthalpy is less than zero, or the magnesium hydride hydrogen production assistants are alkali metal hydroxides of which the dissolution enthalpy is less than zero; 0-20 parts by weight of the assistant for preparing the hydrogen from the magnesium hydride and MgH2 form the magnesium hydride hydrogen production mixed reagent. The method has the advantages that the technological process is simple, the cost is low, the starting time is short, the hydrogen preparation efficiency is high, environmental protection and no pollution are achieved, and the batch production is easy.
Owner:武汉市能智达科技有限公司
Micro-nano magnesium hydride antibacterial and anti-inflammatory wound dressing
PendingCN110559467AAvoid stickinessHas anti-inflammatory propertiesPharmaceutical delivery mechanismAbsorbent padsMicro nanoBiocompatibility Testing
The invention discloses a micro-nano magnesium hydride antibacterial and anti-inflammatory wound dressing. The wound dressing comprises an effective antibacterial and anti-inflammatory component magnesium hydride MgH2 micro-nano powder, an auxiliary material and a dressing matrix, and magnesium hydride micro-nano powder is uniformly loaded and coated on the surface and / or inside the dressing matrix. The magnesium hydride micro-nano powder absorbs moisture immediately when encountering a wound exudate, reacts and is converted into micro-nano magnesium hydroxide particles, meanwhile, local alkalinity is caused, and the magnesium hydride micro-nano powder has broad-spectrum bactericidal activity without illumination induction and good biocompatibility. Meanwhile, hydrogen released by the reaction has non-toxic and anti-inflammatory characteristics and has a certain effect of preventing wound adhesion. The dressing is convenient to store and use, safe, environment-friendly, antibacterial,anti-inflammatory and anti-infection, and is beneficial to wound repair.
Owner:SHANGHAI JIAO TONG UNIV
Metal oxide and porous material composite hydrogen storage material and preparation method thereof
PendingCN110963461AAccelerate the rate of hydrogenationSmall particle sizeHydrogenHydrogen adsorptionHigh surface area
The invention belongs to the technical field of hydrogen storage materials and particularly relates to a metal oxide and porous material composite hydrogen storage material and a preparation method thereof. The metal oxide and porous material composite hydrogen storage material is formed by compounding magnesium hydride, a porous material and a metal oxide, and the weight ratio of the magnesium hydride to the porous material to the metal oxide is (100-10): (5-0.1): (1-0.05). The magnesium hydride is taken as a base material, and metal oxide particles are mixed and grinded to obtain an activated magnesium hydride material; and the obtained activated magnesium hydride material is mixed with the porous material, and ball milling or compression is carried out to obtain the composite hydrogen storage material. According to the material and the preparation method, nanoscale metal oxide particles are added and compounded with the porous material, so that the hydrogenation speed of magnesium-based composite powder in the hydrogen charging ball milling process can be increased, and meanwhile, the hydrogen storage material has good characteristics in the aspects of hydrogen adsorption and desorption kinetics in combination with the properties such as excellent pore channels and high surface area of the porous material.
Owner:世能氢电科技有限公司 +1
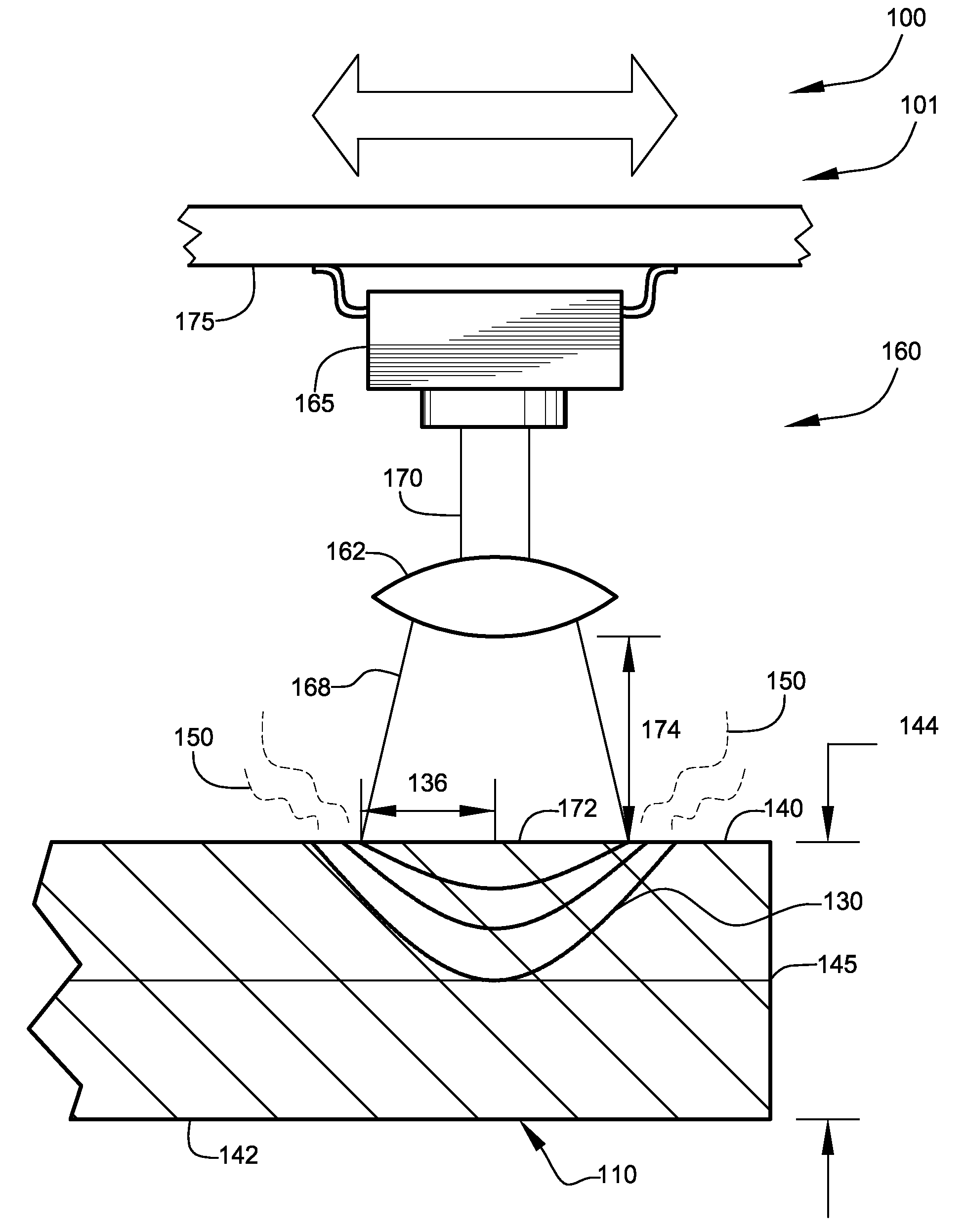
Method for Fabricating Magnesium-Based Hydrogen Storage Material
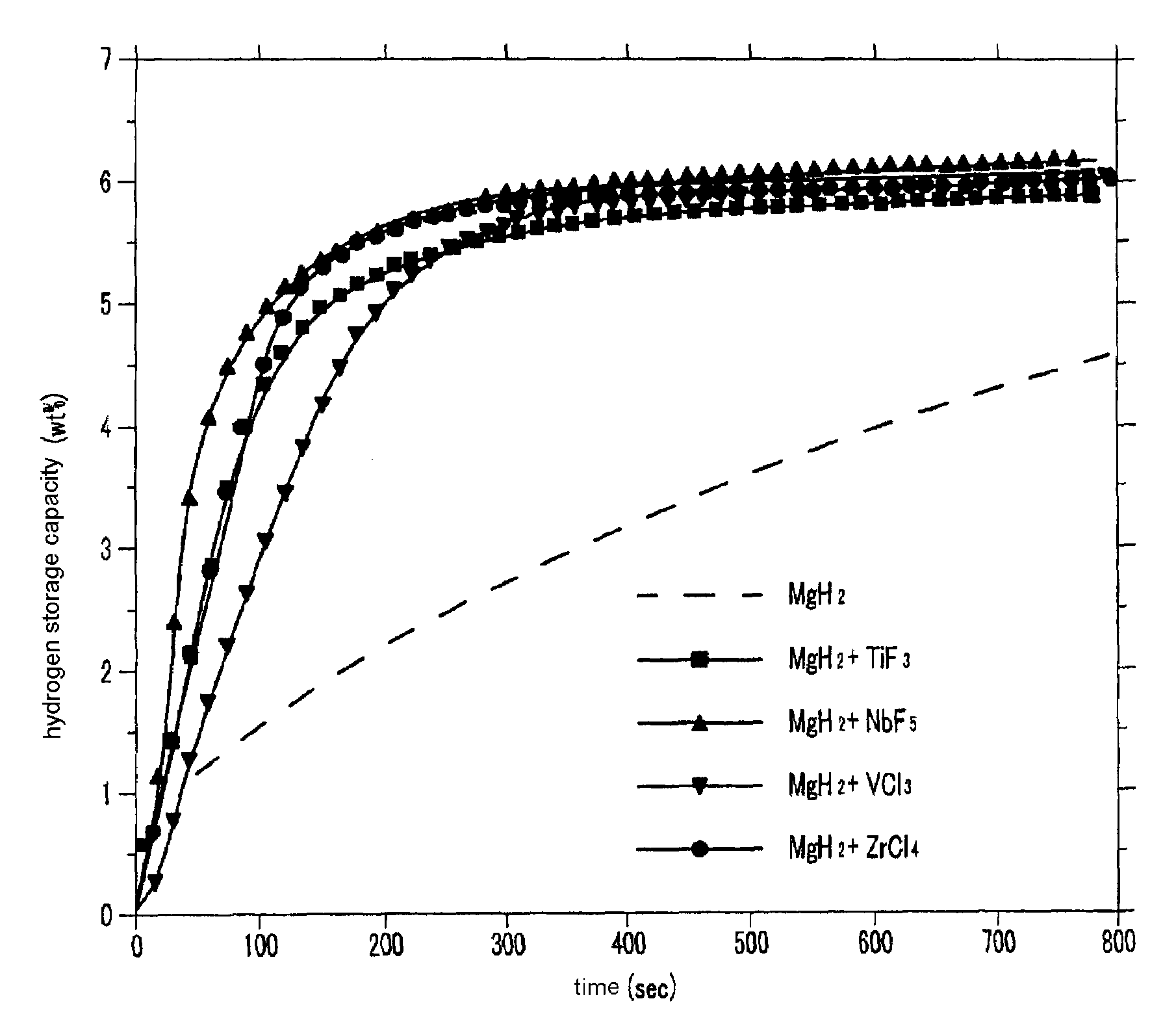
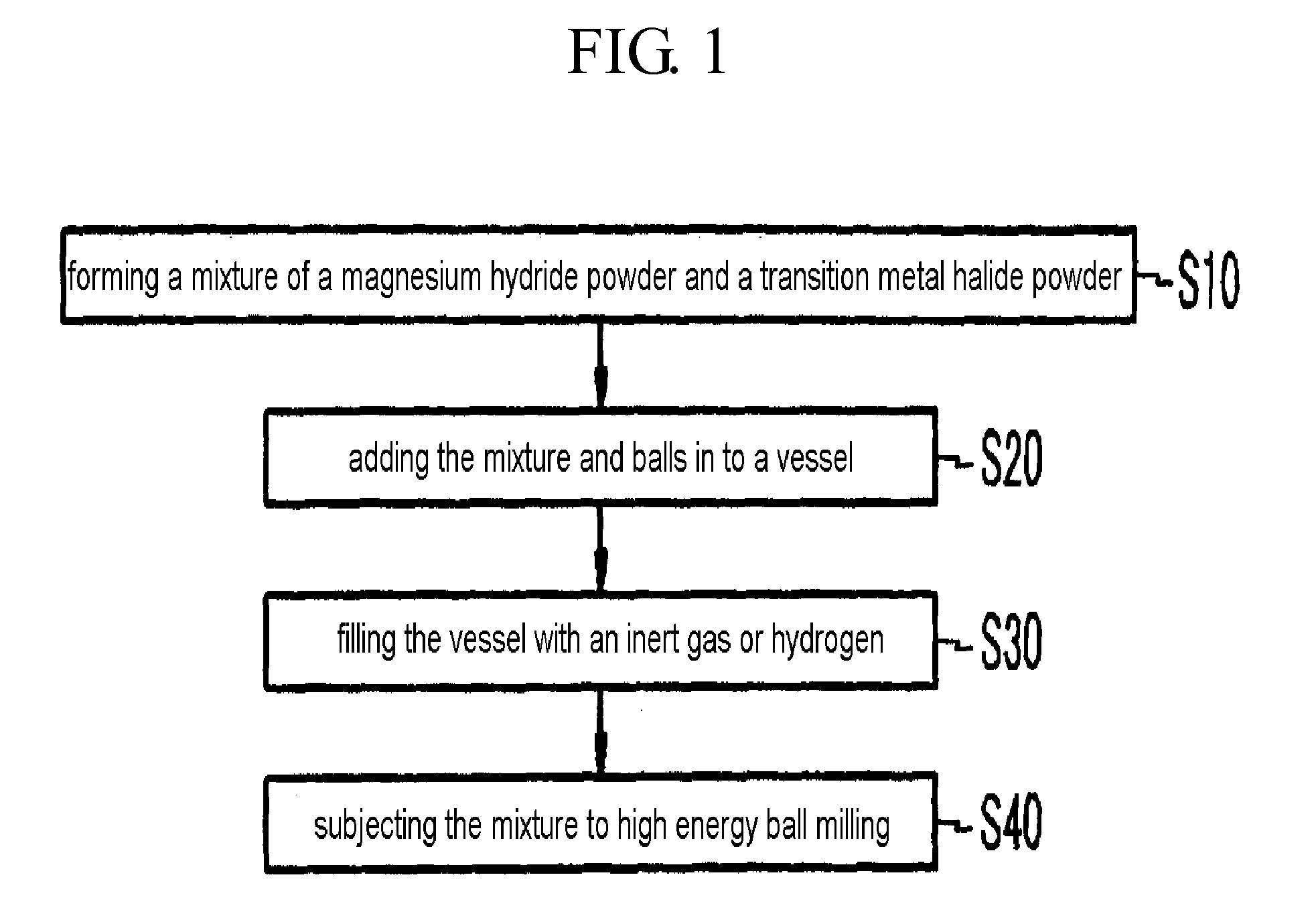
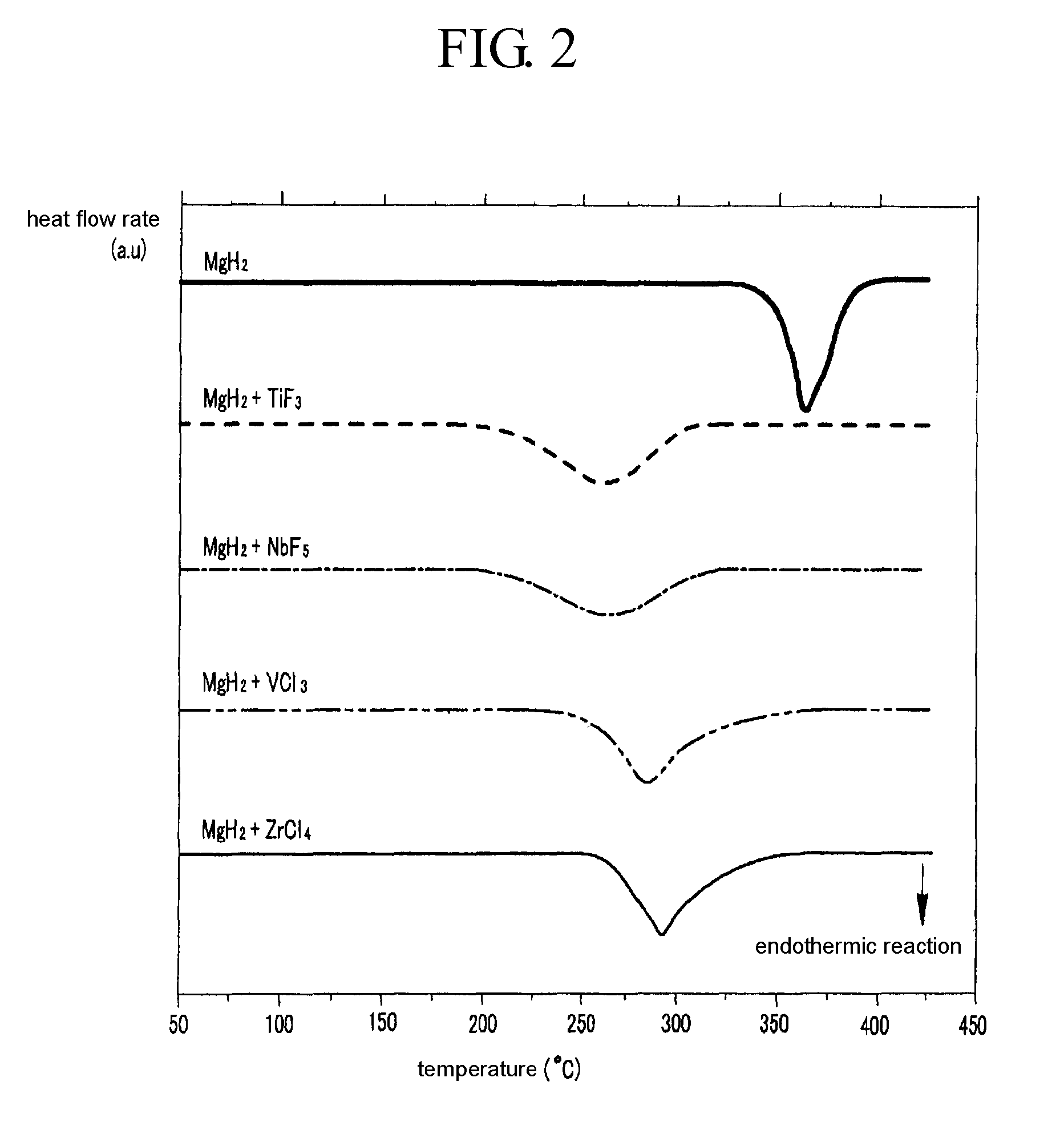
InactiveUS20080111105A1Maximize capacityIncrease ratingsHydrogenOther chemical processesHigh energyHalide
A method for fabricating a magnesium-based hydrogen storage material according to the present invention comprises a) forming a mixture of a magnesium hydride powder and a transition metal halide powder, b) adding the mixture and balls into a vessel, c) filling the vessel with an inert gas or hydrogen, and d) subjecting the mixture to high energy ball milling.
Owner:KOREA INST OF SCI & TECH
Preparation method of in-situ growth nano magnesium hydride loaded high specific surface material
ActiveCN106865497ALower operating temperatureFast hydrogen absorption and desorption rateHydrogenAlkali/alkaline-earth/beryllium/magnesium hydridesOperating temperatureHydrogen absorption
The invention relates to a preparation method of an in-situ growth nano magnesium hydride loaded high specific surface material. An alkali metal hydride, magnesium halide and a support material are utilized for in situ synthesis of the material under a ball milling condition. The invention has the technical effects that: magnesium hydride is generated in situ on the support material surface through replacement reaction, the nano-composite hydrogen storage material with low operating temperature and fast hydrogen absorption and desorption rate is prepared under a mild condition, the problems of harsh preparation conditions, large product particle size and the like in previous nano magnesium hydride preparation, and the thermodynamic and dynamic performance of the magnesium hydride hydrogen storage material can be improved.
Owner:NANKAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com