Method for preparing sodium borohydride by chemical mechanical mechanics method
A technology of sodium borohydride and chemical machinery, applied in the direction of borane/diborane hydride, etc., can solve the problems of sodium borohydride environmental pollution, harsh operating conditions, etc., achieve temperature reduction, low raw material cost, simple and safe process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
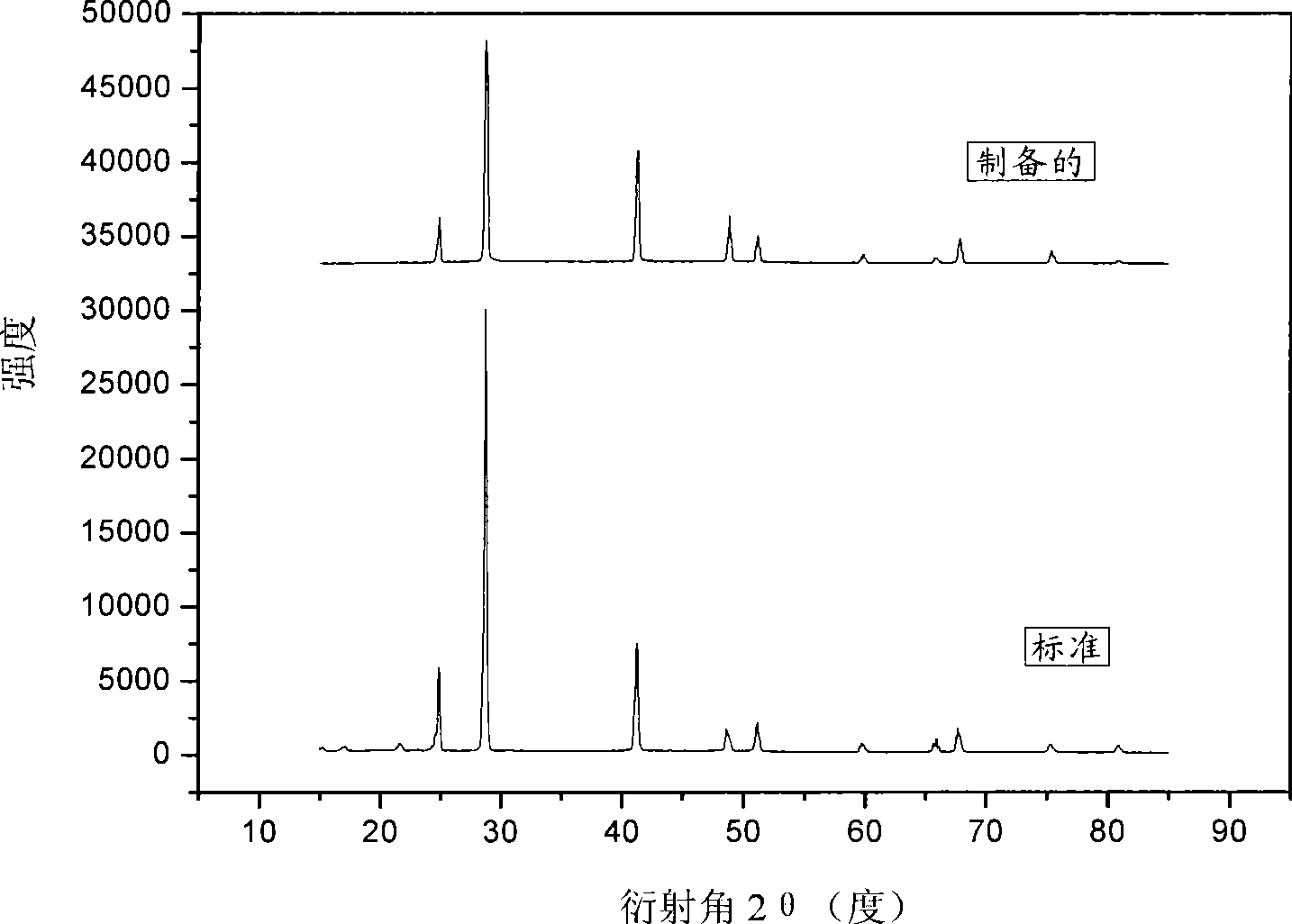
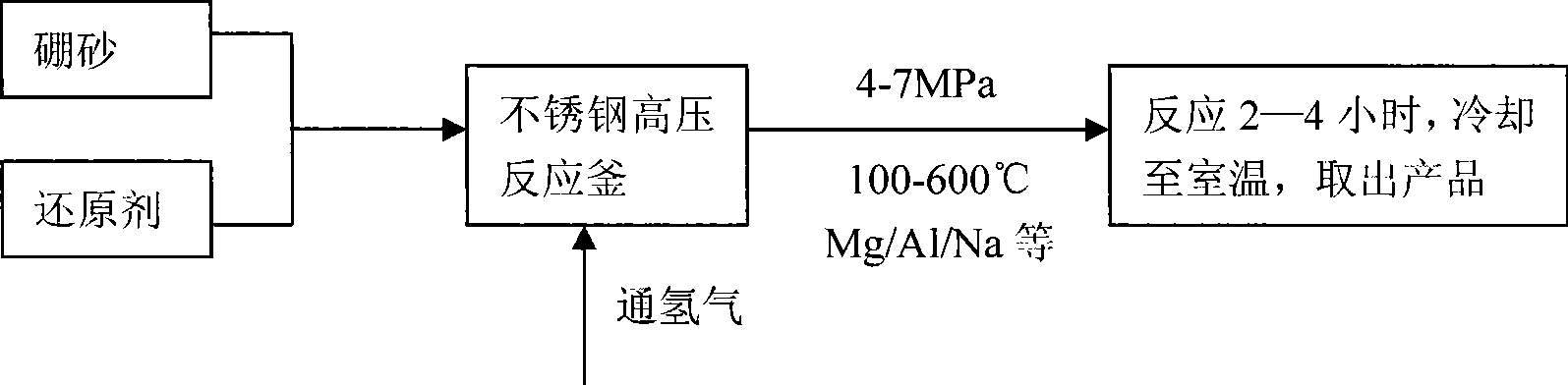
Image
Examples
Embodiment 1
[0023] 1. Raw material handling
[0024] Dry the borax in a drying oven at 500±30°C for more than 4 hours, remove all the crystal water, then ball mill and grind it, and take it through 150 meshes for later use.
[0025] 2. Synthesis of magnesium hydride
[0026] Weigh 60g of magnesium powder (over 200 mesh) into the autoclave (GCF-1 autoclave), seal the autoclave, fill it with argon and vacuumize, repeat 3 times, then turn on the power and heat the autoclave to 300°C, then fill it with hydrogen To 6.0MPa, react for 24h. After the reaction, stop heating, cool to room temperature, release the pressure to normal pressure, and fill with argon to protect the product.
[0027] 3. Synthesis of sodium borohydride
[0028] Weigh 2.0g of anhydrous borax, 1.3g of magnesium hydride and 0.2g of anhydrous sodium carbonate, put them into a ball mill tank (QM-3A high-speed vibrating ball mill), and put them into Steel balls, then evacuated, filled with argon, repeated three times, finall...
Embodiment 2
[0031] The difference from Example 1 is:
[0032] The magnesium powder is mechanically ground at 200°C and the hydrogen pressure is 4.5MPa to react with hydrogen to produce magnesium hydride; put 2.0g of anhydrous borax, 0.3g of anhydrous sodium carbonate, and 1.3g of magnesium hydride into the ball milling equipment, Keep the hydrogen pressure at 100kPa and the mass ratio of ball to material at 60:1, and the grinding time is 2h.
[0033] The yield is 27%, and the purity is greater than 97% (calculated by active hydrogen).
Embodiment 3
[0035] The difference from Example 1 is:
[0036] Take 100 meshes of dry anhydrous borax for later use, carry out mechanical grinding on magnesium powder at 300°C and hydrogen pressure of 3 MPa to react with hydrogen to produce magnesium hydride; prepare 2.0 g of anhydrous borax, 0.1 g of anhydrous sodium carbonate, and magnesium hydride 1.1g, put it into the ball milling equipment, keep the hydrogen pressure 100kPa, the mass ratio of ball to material 50:1 and the grinding time 1.5h.
[0037] The yield is 18%, and the purity is greater than 97% (calculated by active hydrogen).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com