Method and device for preparing magnesium hydride
A magnesium hydride and hydrogen technology, applied in the direction of alkali metal/alkaline earth metal/beryllium/magnesium hydride, etc., can solve the problems of coarse grain size, high price, slow hydrogen absorption and desorption kinetics, etc., and achieve simple process and high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
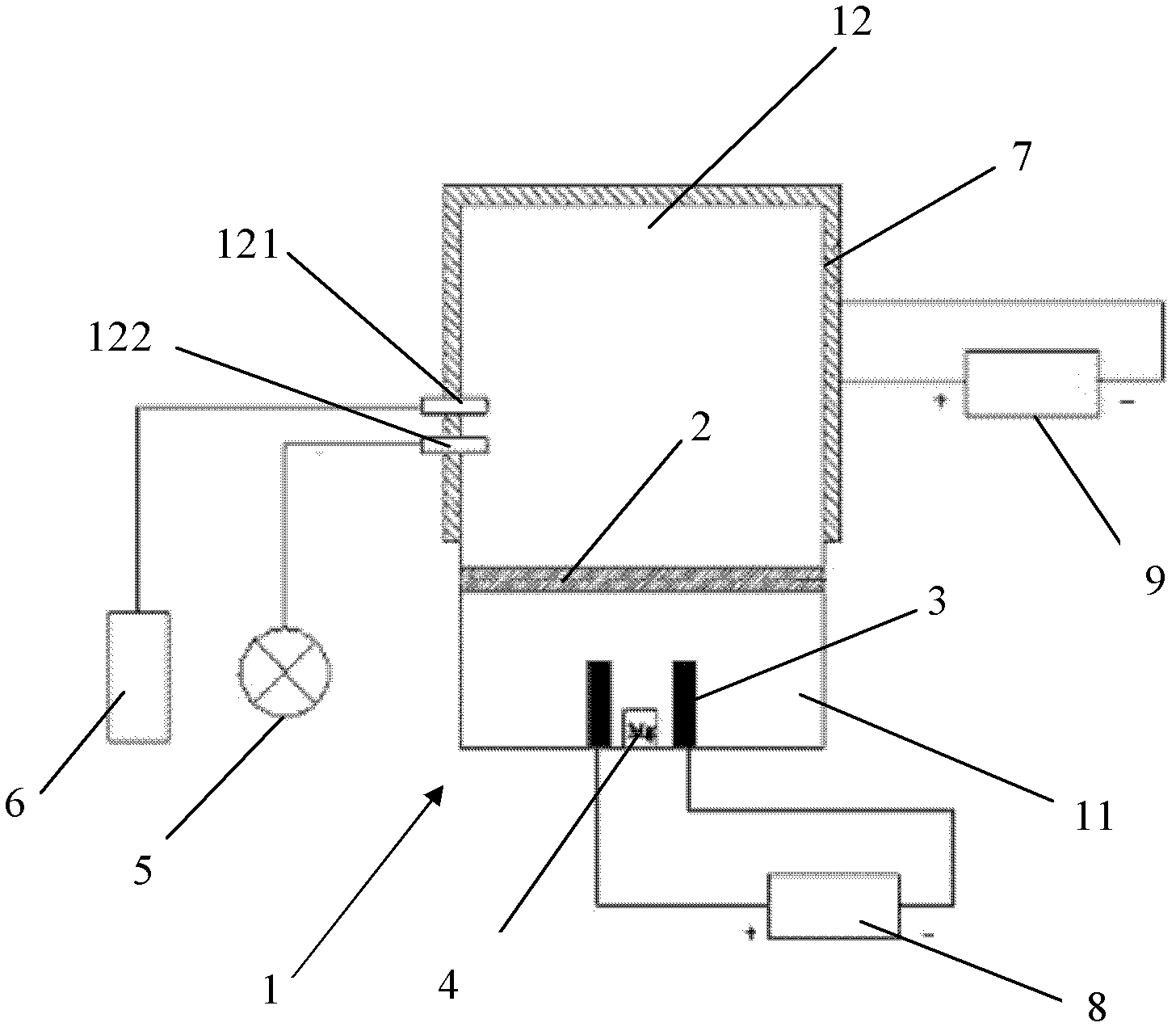
[0042] The method for preparing magnesium hydride of the present invention generally comprises the following steps:
[0043] a) vacuumize the reaction vessel;
[0044] b) In the vacuum environment of the reaction vessel, the magnesium block is heated and evaporated into magnesium vapor, and part of the magnesium vapor is condensed into magnesium powder on the upper part of the reaction vessel;
[0045] c) charging hydrogen into the reaction vessel;
[0046] d) heating, and insulation, allowing magnesium powder, magnesium vapor and hydrogen to react;
[0047] e) cooling, allowing the magnesium hydride generated to be deposited on the inner wall of the reaction vessel;
[0048] f) Passivation, collecting and obtaining magnesium hydride.
[0049] In step a), the reaction vessel is evacuated to 10 -3 -10 -2 Pa. In step b), the magnesium block is heated to 650-750° C. to evaporate into magnesium vapor. In step c), hydrogen is charged until the pressure is 5-8 MPa. In step d...
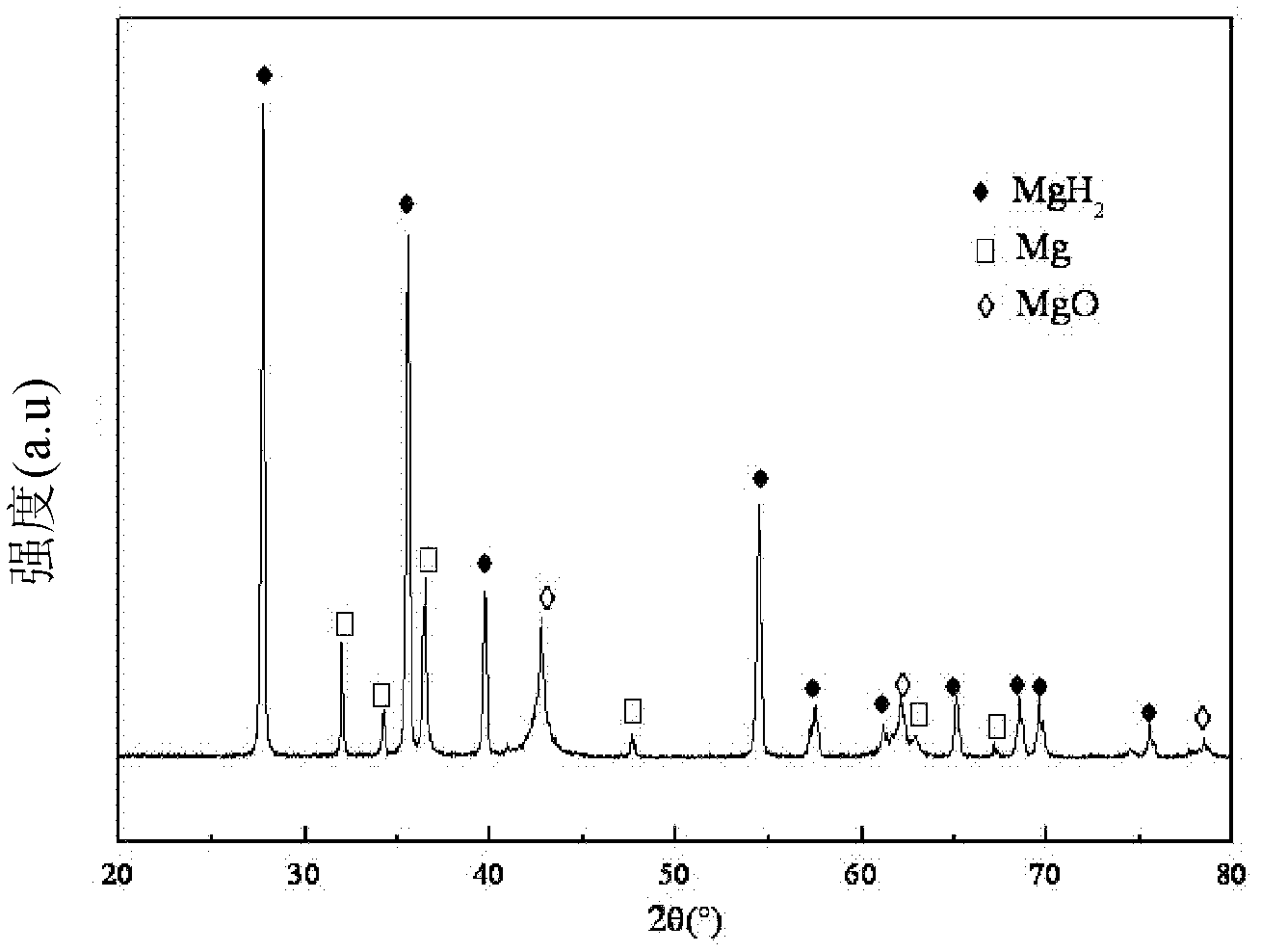
Embodiment 1
[0063] 1) Open the lid of the reaction kettle 1 and the plug-in valve 2, put the pure Mg block into a ceramic crucible (caliber slightly smaller than the first heating coil 3), and then place it in the first heating coil 3 of the lower part 11 of the reaction kettle 1;
[0064] 2) Close the lid, the air inlet 121, the air inlet and outlet and the air outlet 122 of the reactor 1, and seal the reactor 1;
[0065] 3) Open vacuum pump 5 and air outlet 122, vacuumize to 10 -2 Pa, then close the vacuum pump 5 and the air outlet 122;
[0066] 4) Turn on the first control power supply 8, the first heating coil 3 works, heat to 650°C to evaporate the Mg block, and the Mg vapor will deposit on the inner wall of the reaction kettle when it is cooled to 650°C. The time is 5 hours;
[0067] 5) Close the plugboard valve 2 and the first control power supply 8, charge H 2 to 6MPa;
[0068] 6) Turn on the second control power supply 9, the second heating coil works, heat to 350°C and then ...
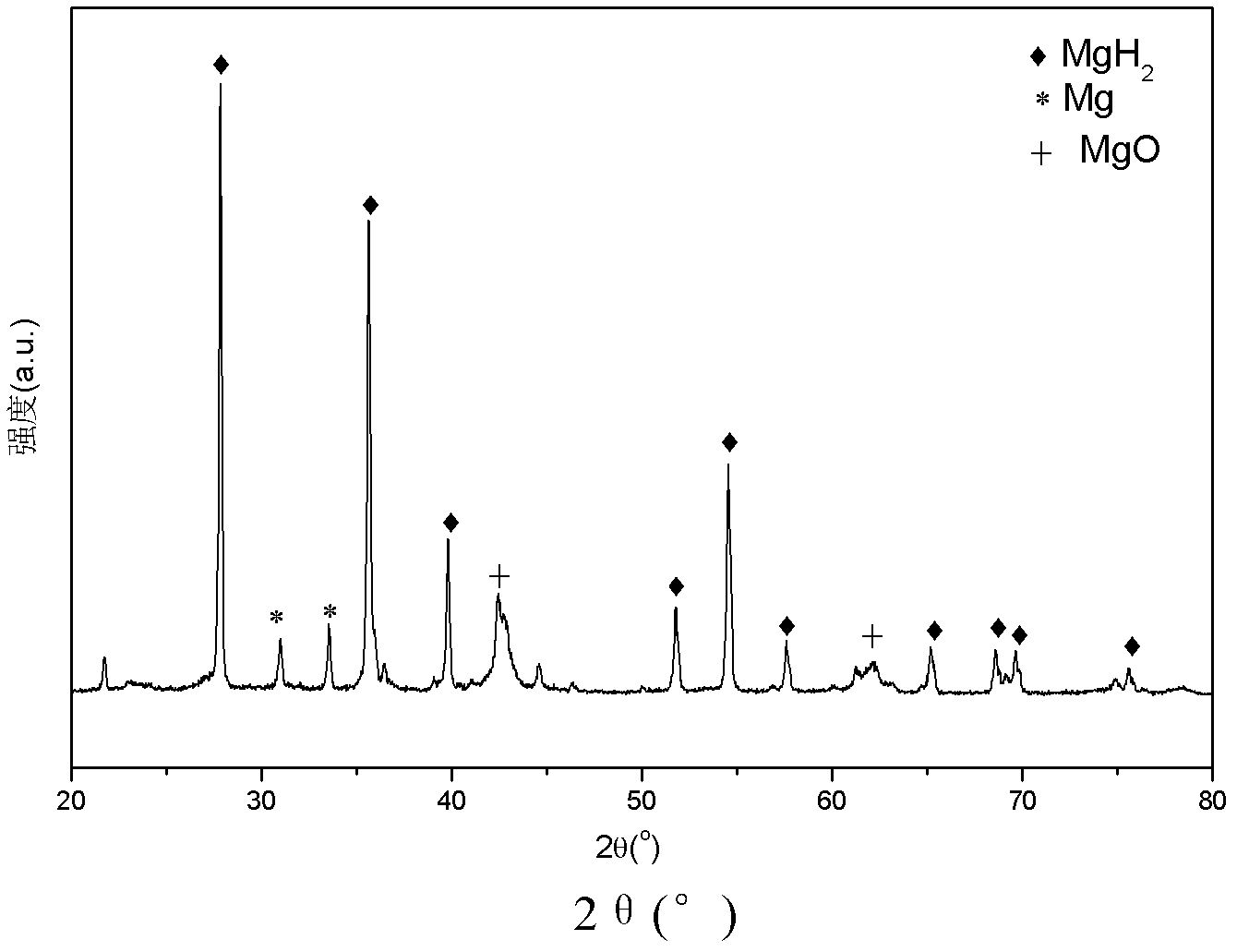
Embodiment 2
[0073] 1) Open the lid of the reaction kettle 1 and the plug-in valve 2, put the pure Mg block into a ceramic crucible (caliber slightly smaller than the first heating coil 3), and then place it in the first heating coil 3 of the lower part 11 of the reaction kettle 1;
[0074] 2) Close the lid, the air inlet 121, the air inlet and outlet and the air outlet 122 of the reactor 1, and seal the reactor 1;
[0075] 3) Open vacuum pump 5 and air outlet 122, vacuumize to 10 -2 Pa, then close the vacuum pump 5 and the air outlet 122;
[0076] 4) Turn on the first control power supply 8, the first heating coil 3 works, heat to 700°C to evaporate the Mg block, and the Mg vapor reaches the upper part 12 of the reactor 1 and deposits on the inner wall of the reactor when it cools, and heats to 700°C The time taken is 5 hours;
[0077] 5) Close the plugboard valve 2 and the first control power supply 8, charge H 2 to 8MPa;
[0078] 6) Turn on the second control power supply 9, the sec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com