Patents
Literature
1228results about "Fixed capacity gas holders" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Systems and methods for forming metal oxides using alcohols
InactiveUS7041609B2Reduce decreaseAvoid problemsTransistorSolid-state devicesAlcoholDeposition process
A method of forming (and an apparatus for forming) a metal oxide layer on a substrate, particularly a semiconductor substrate or substrate assembly, using a vapor deposition process, one or more alcohols, and one or more metal-containing precursor compounds.
Owner:MICRON TECH INC
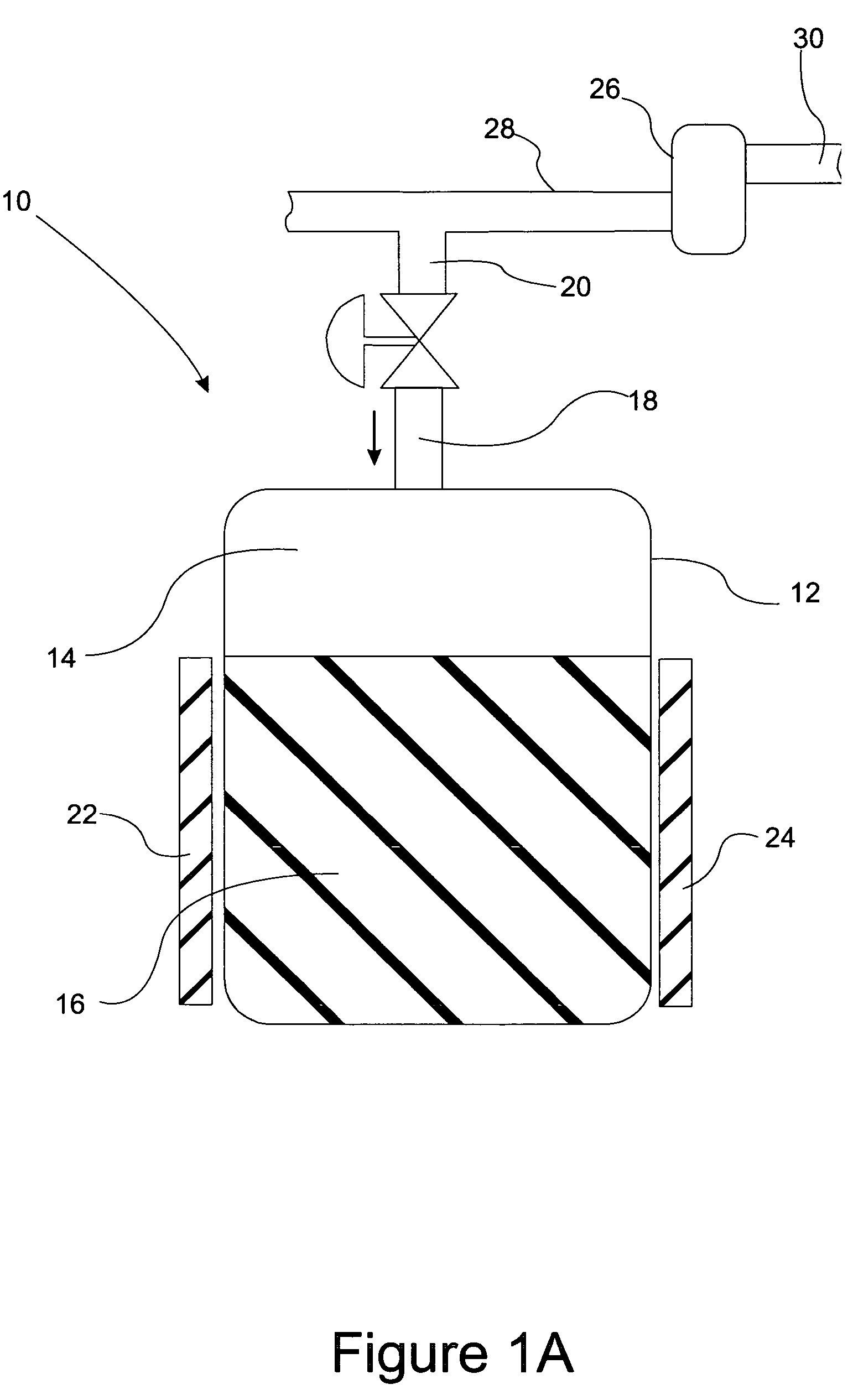
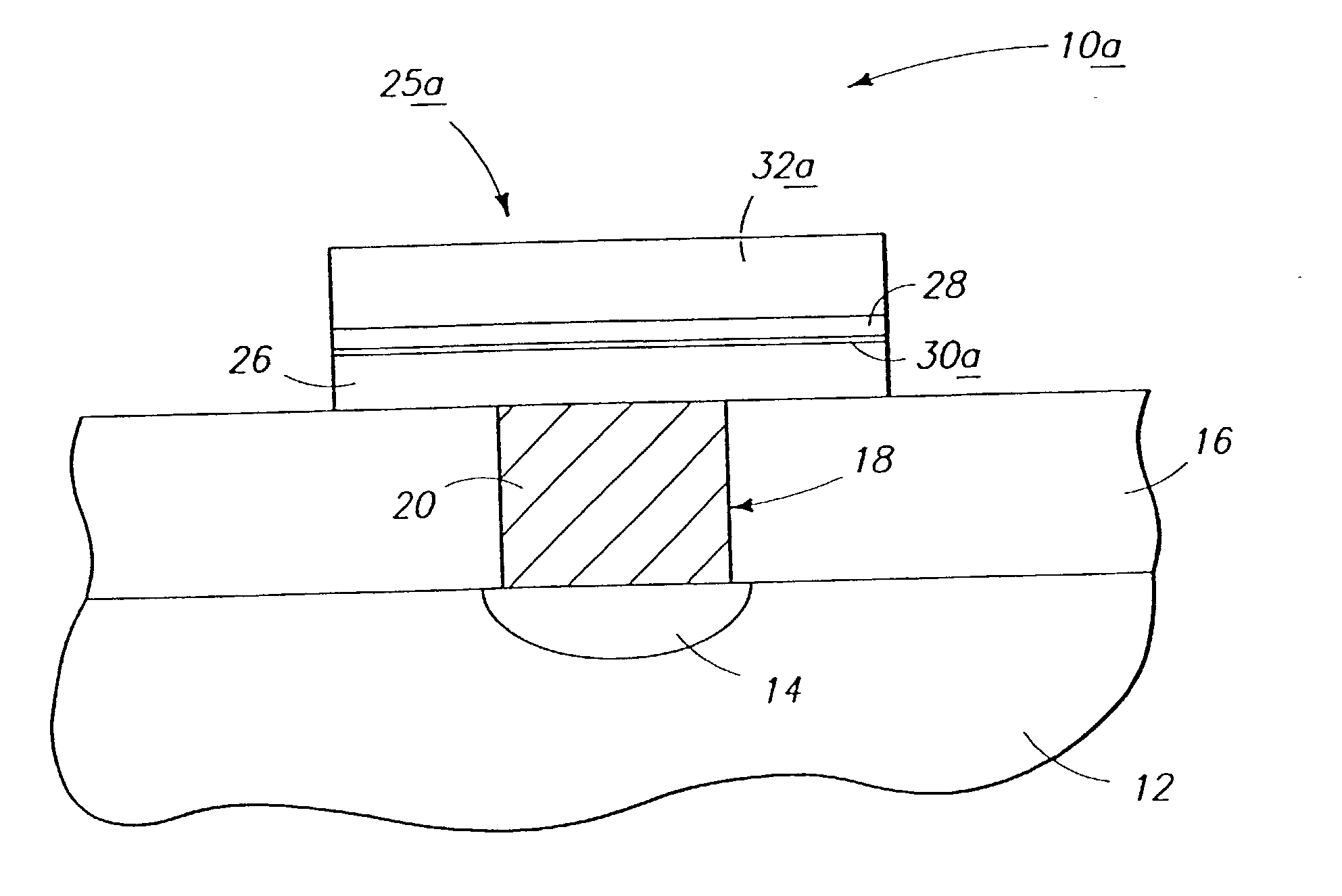
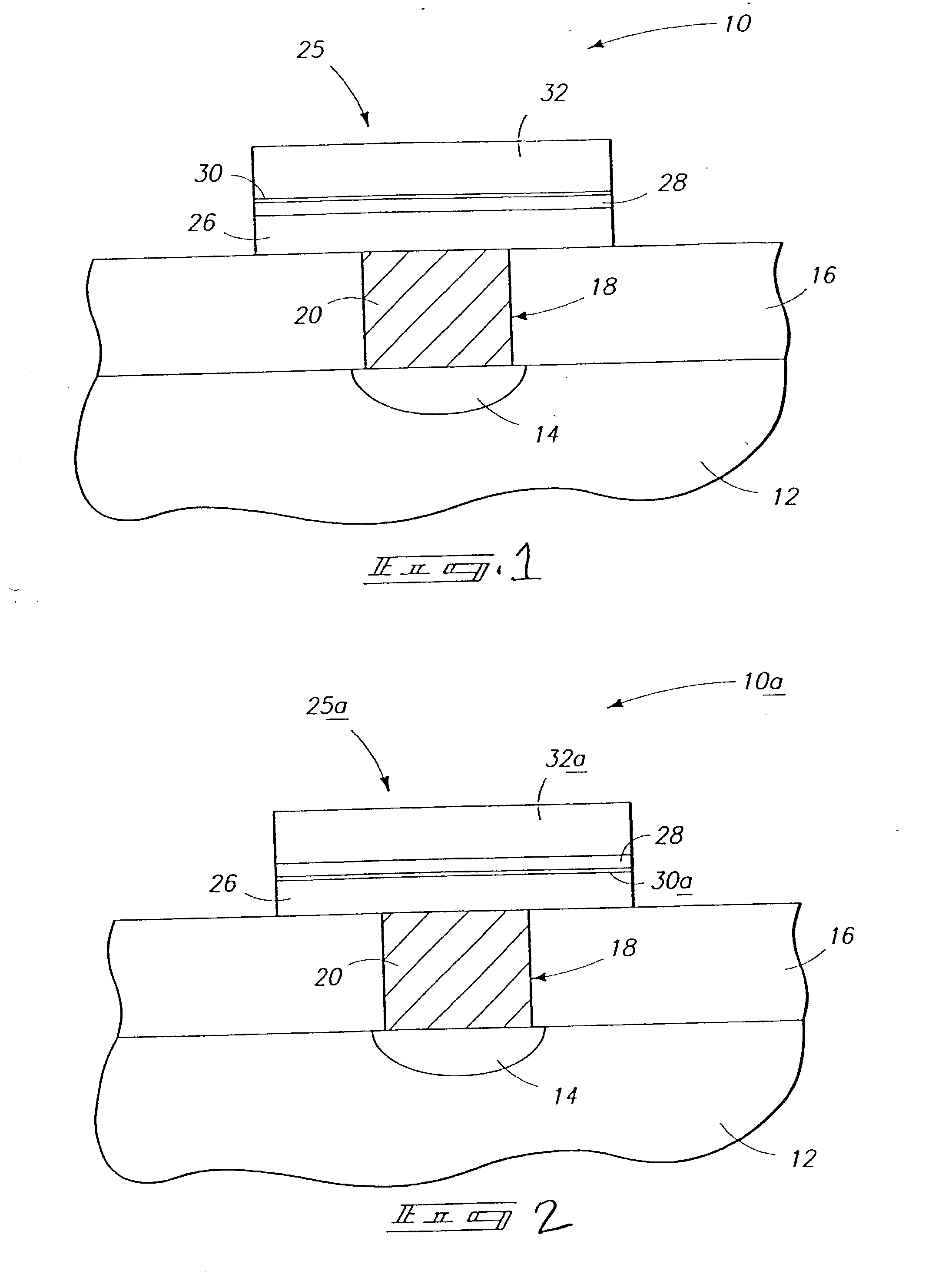
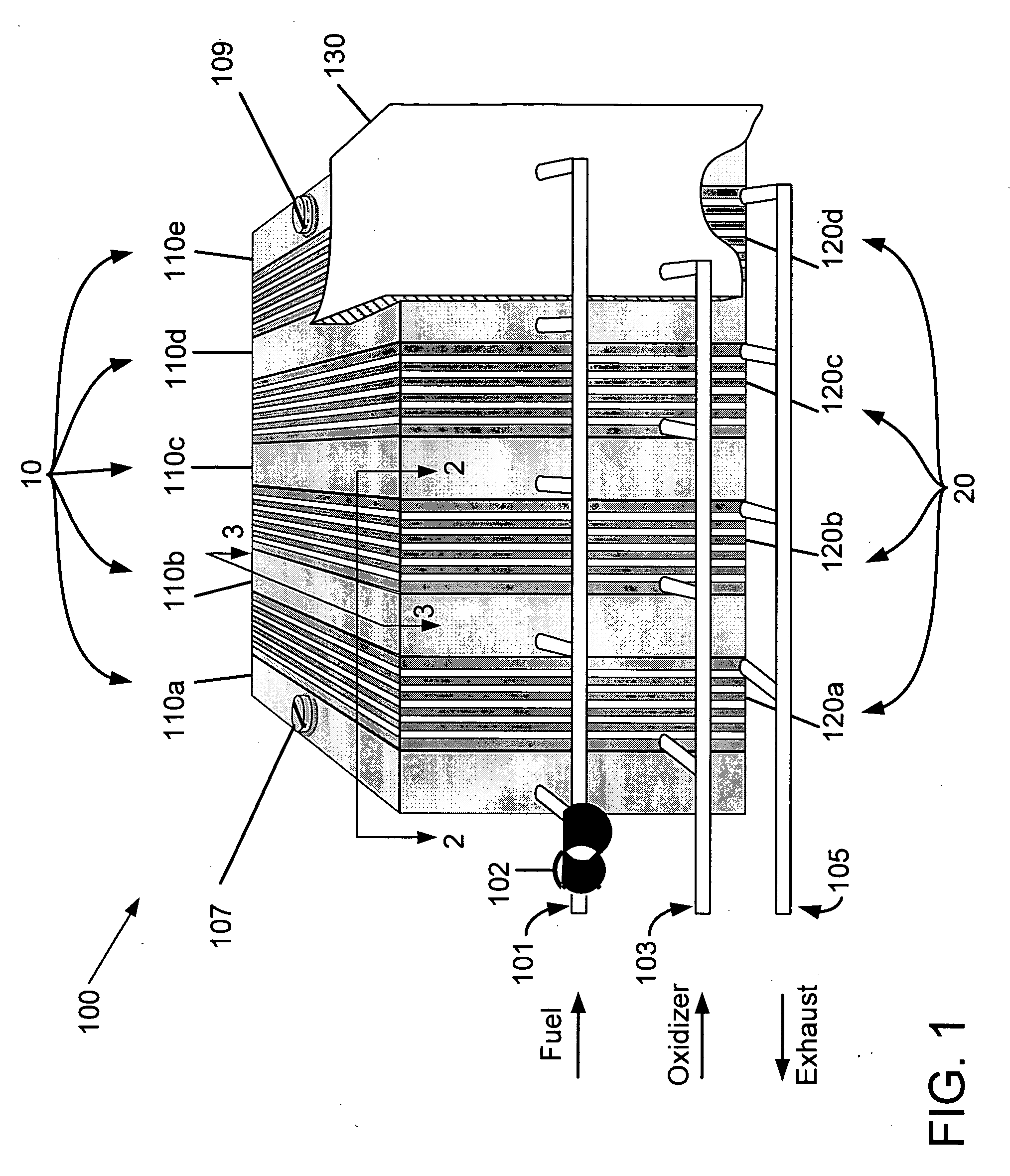
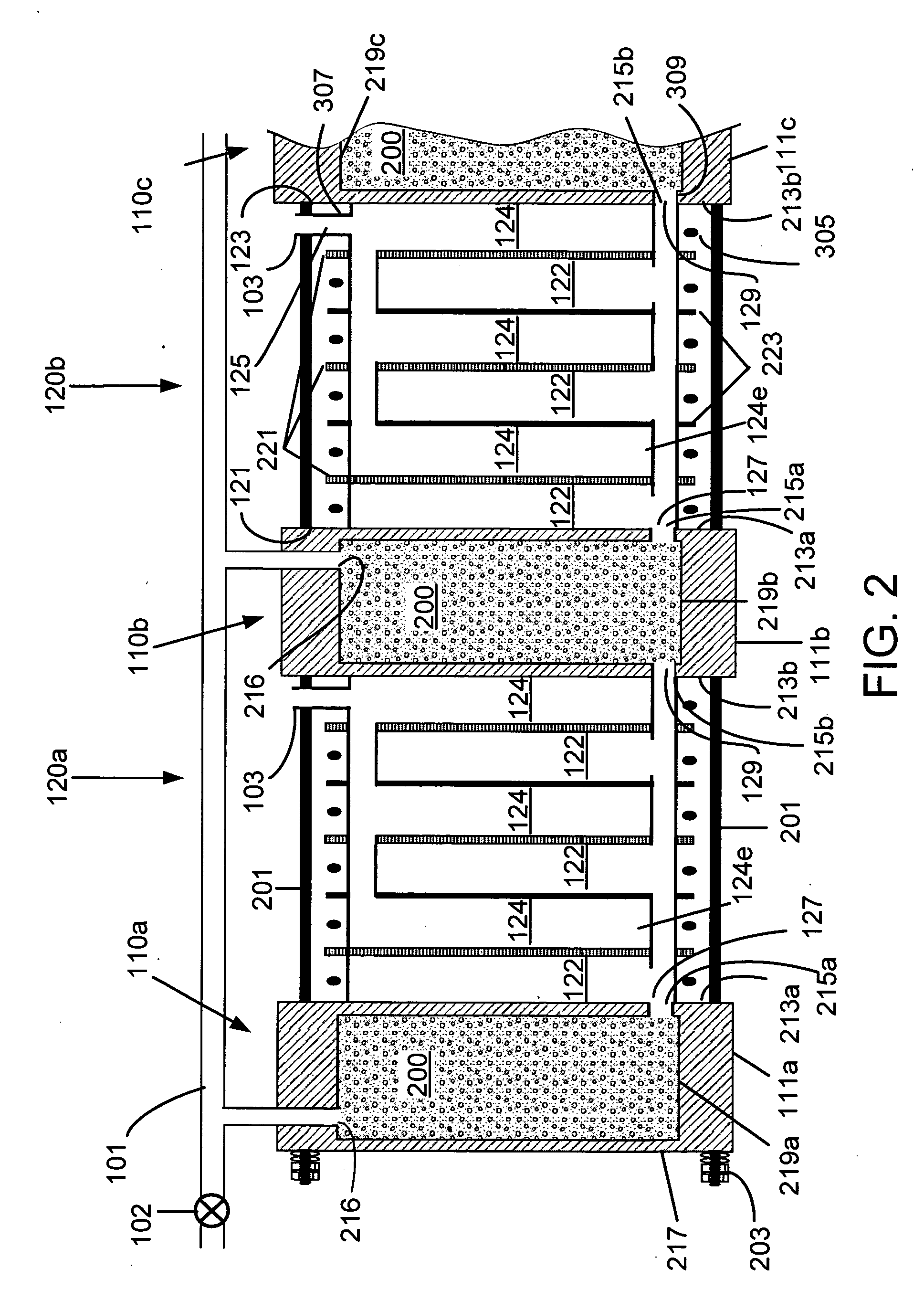
Fuel cartridge with connecting valve
A shut-off valve or connecting valve capable of connecting a fuel supply to a fuel cell is disclosed. The valve comprises a first valve component and a second valve component. Each valve component has an outer housing and a biased slidable member disposed inside the housing forming an internal seal. During the connection process, the two valve components establish an inter-component seal. Afterward, in one suitable embodiment the slidable member moves inward and opens the internal seal in the valve component to establish a flow path. In another embodiment, the slidable member moves inward and exposes a first filler and the first filler abuts a second filler in the other valve component to establish a flow path. In other embodiments, at least one valve component is sized and dimensioned to limit access to the internal seal.
Owner:INTELLIGENT ENERGY LTD
Method and package for storing a pressurized container containing a drug
Owner:GLAXO SMITHKLINE LLC
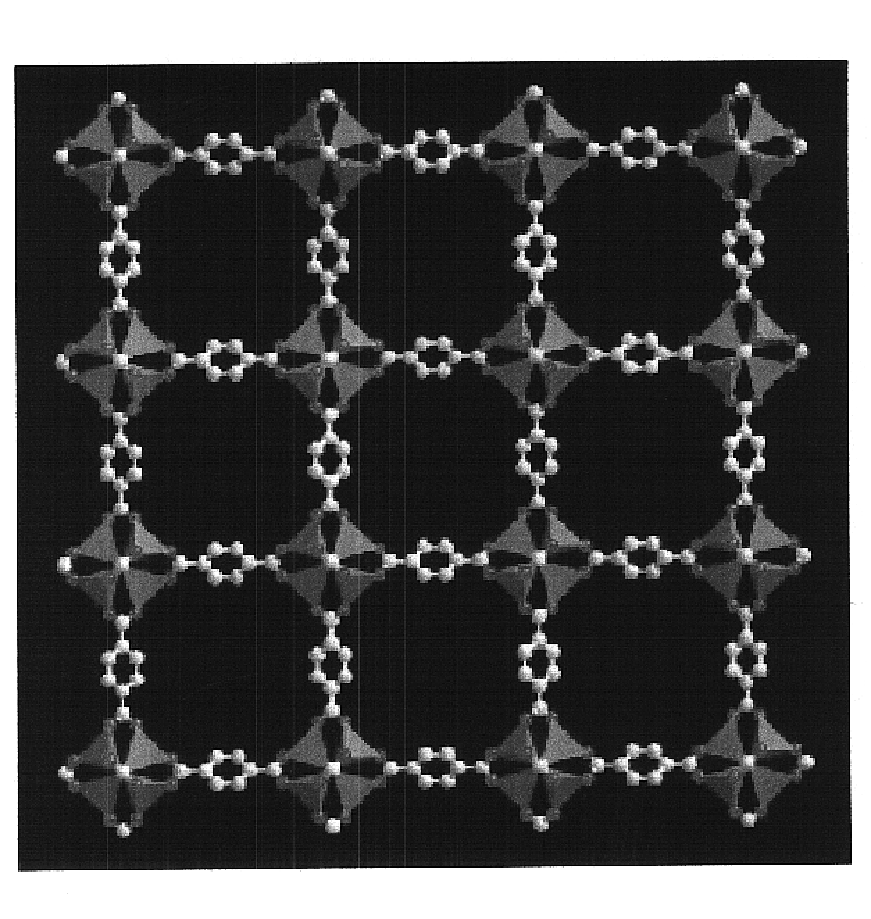
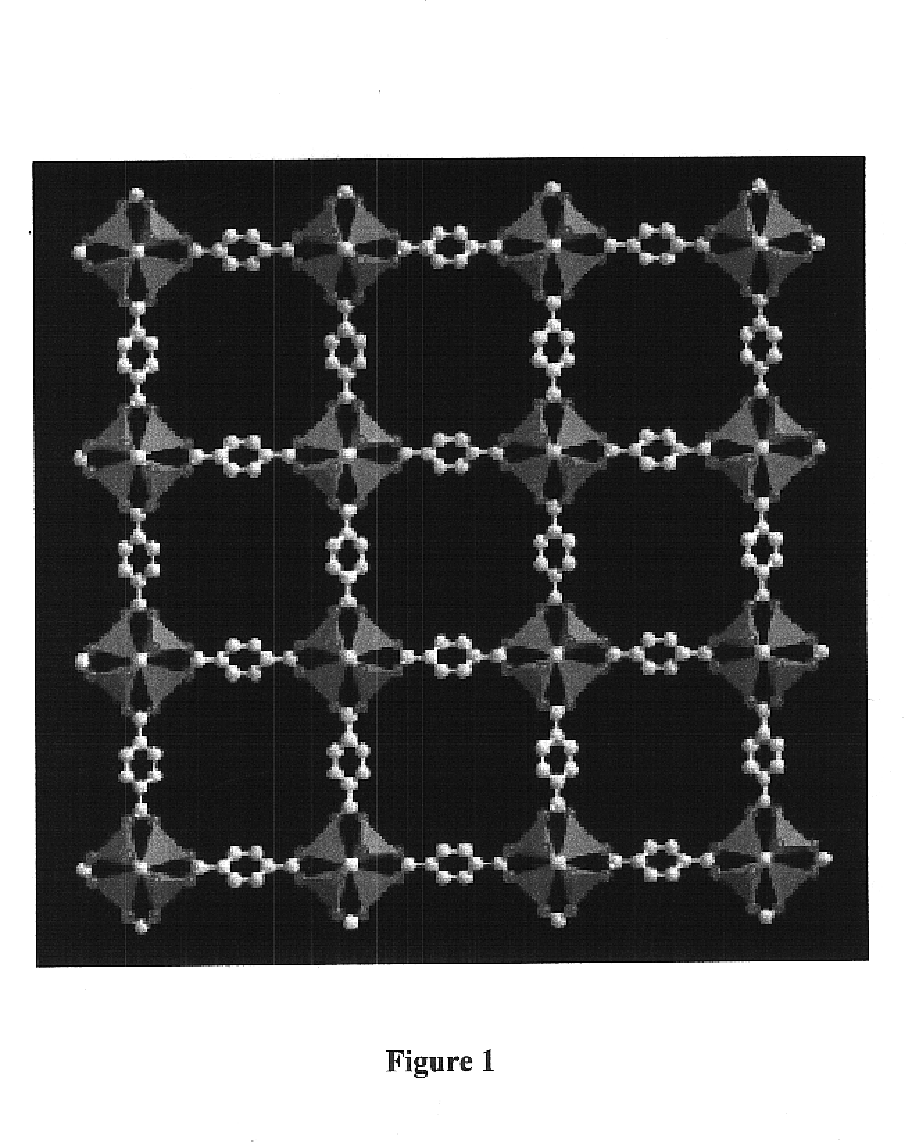
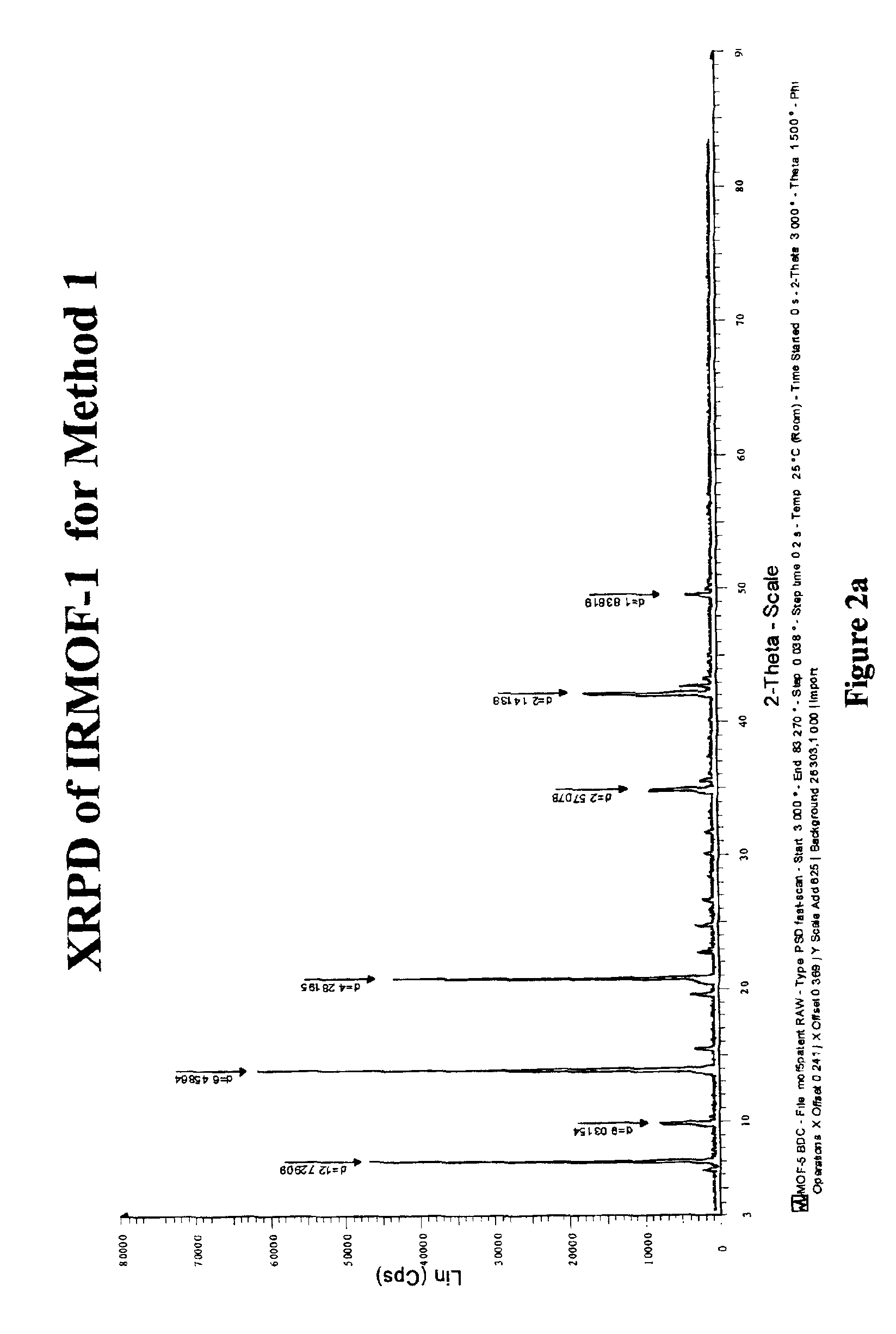
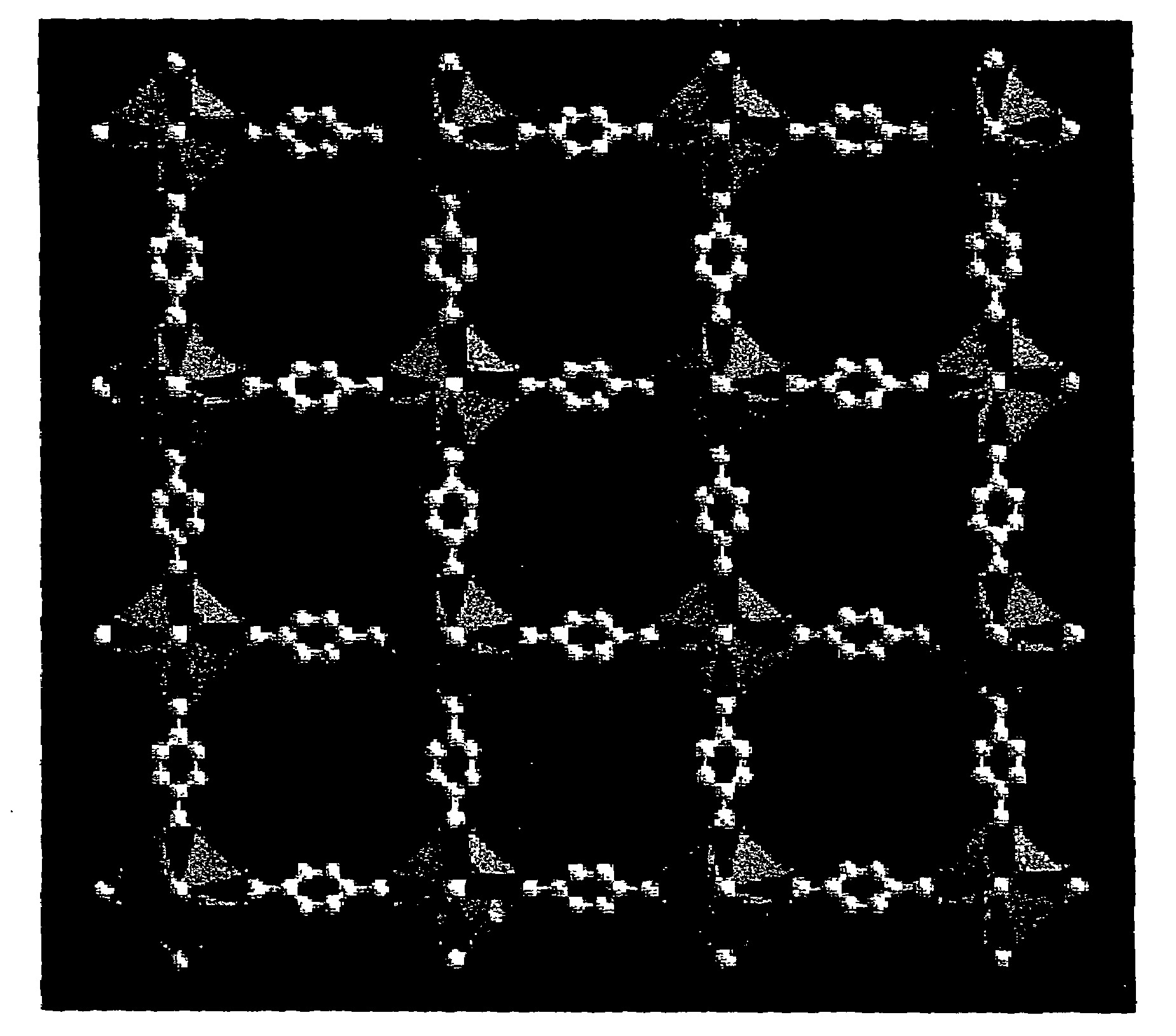
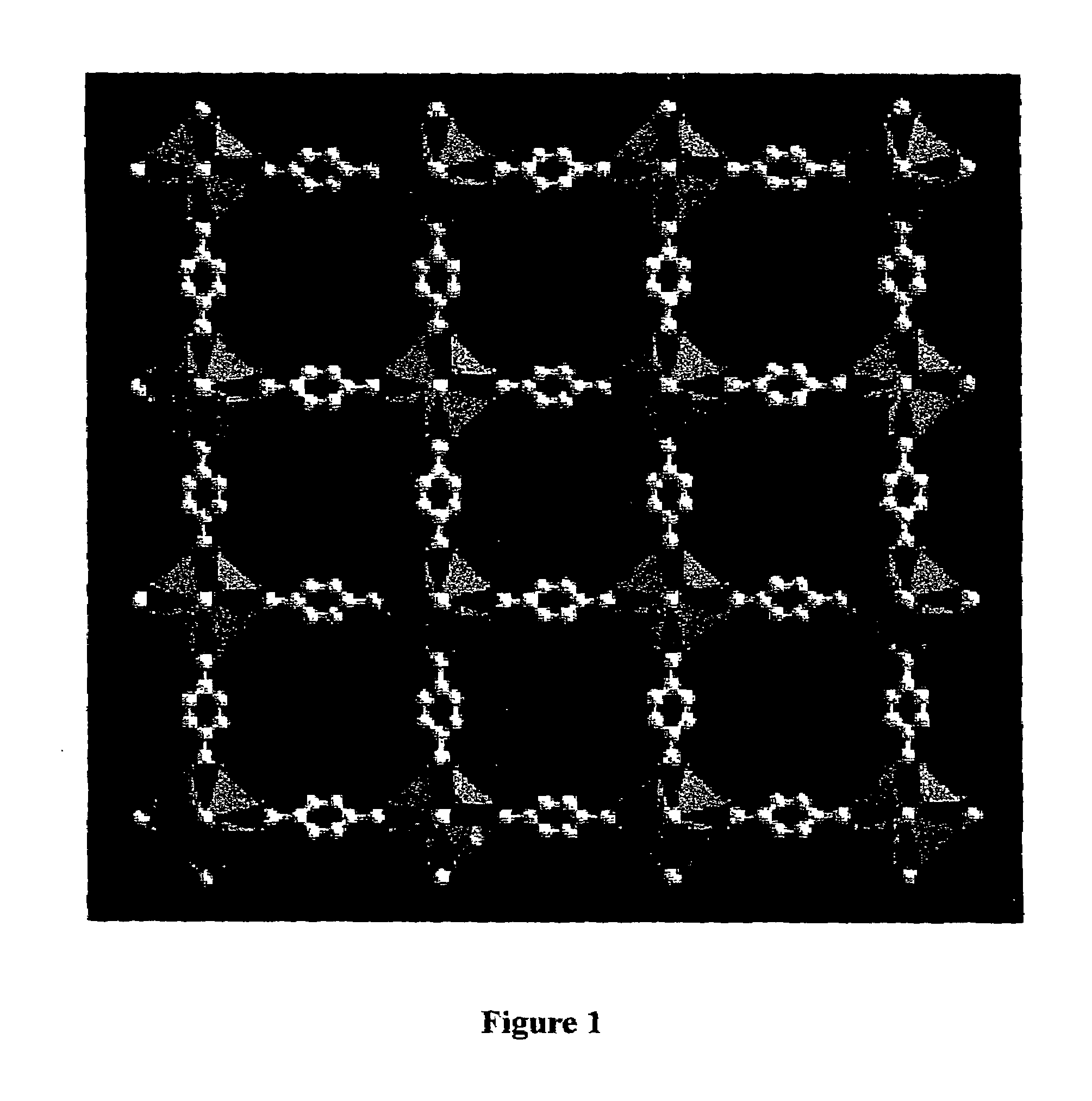
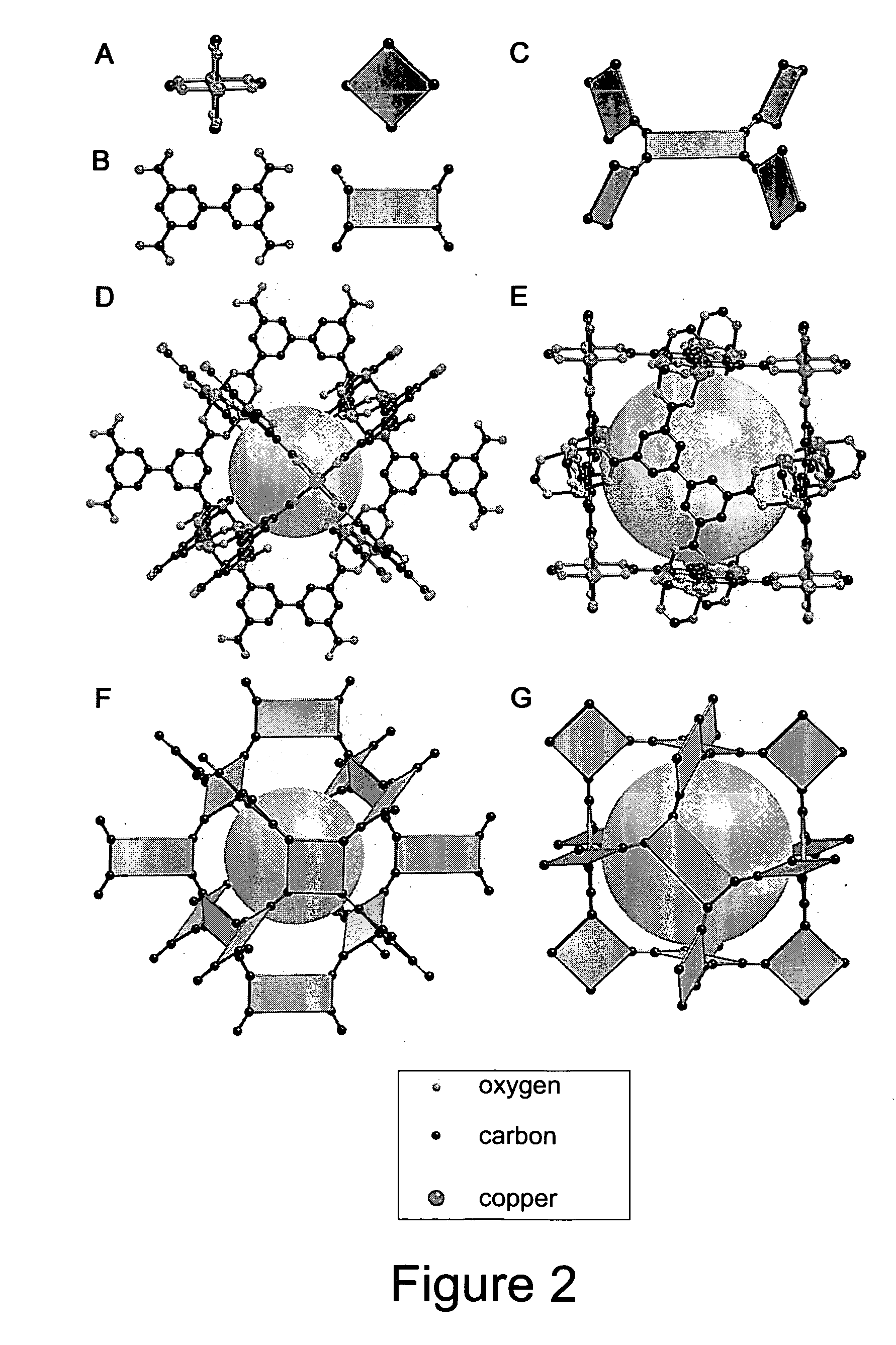
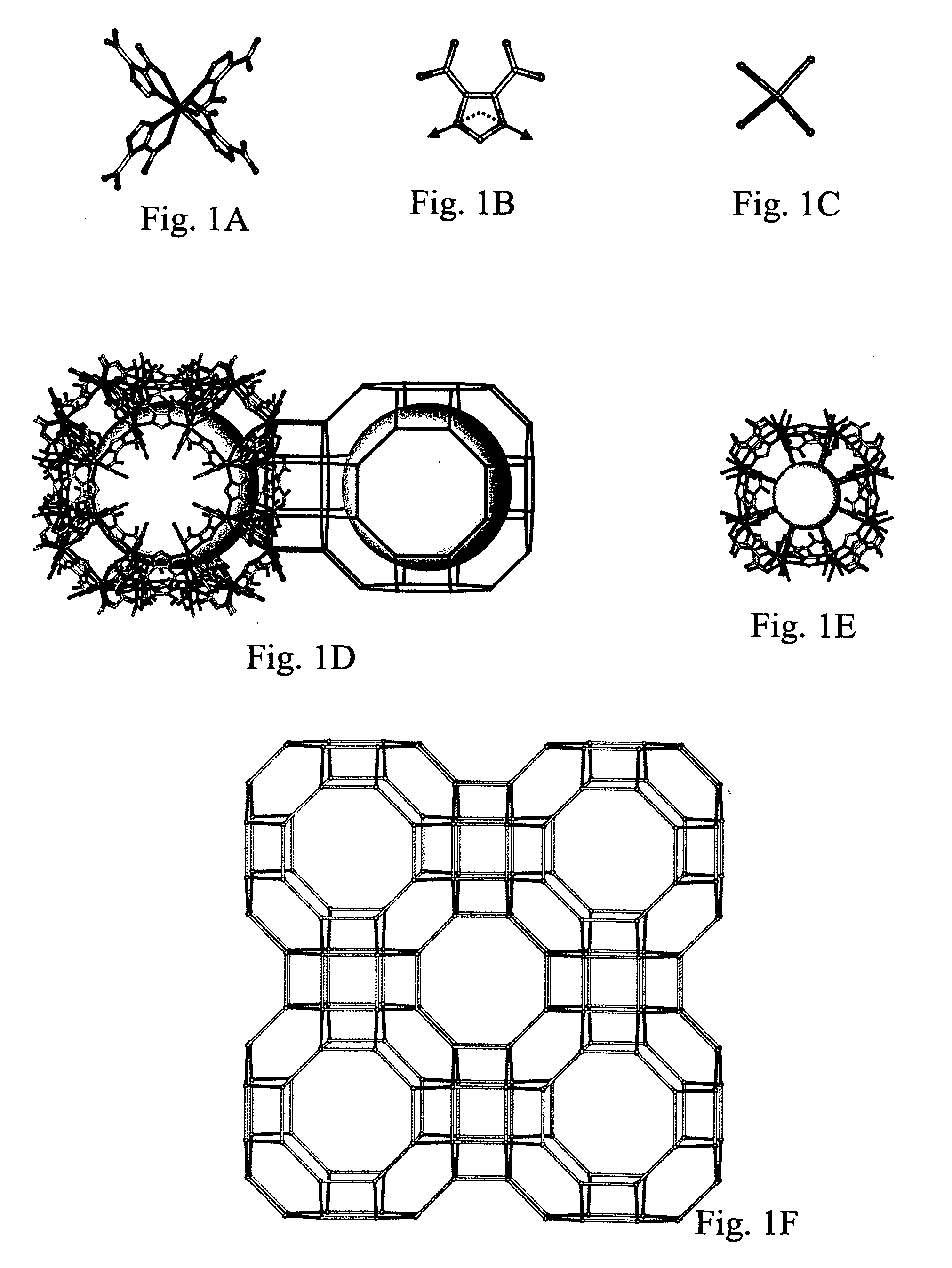
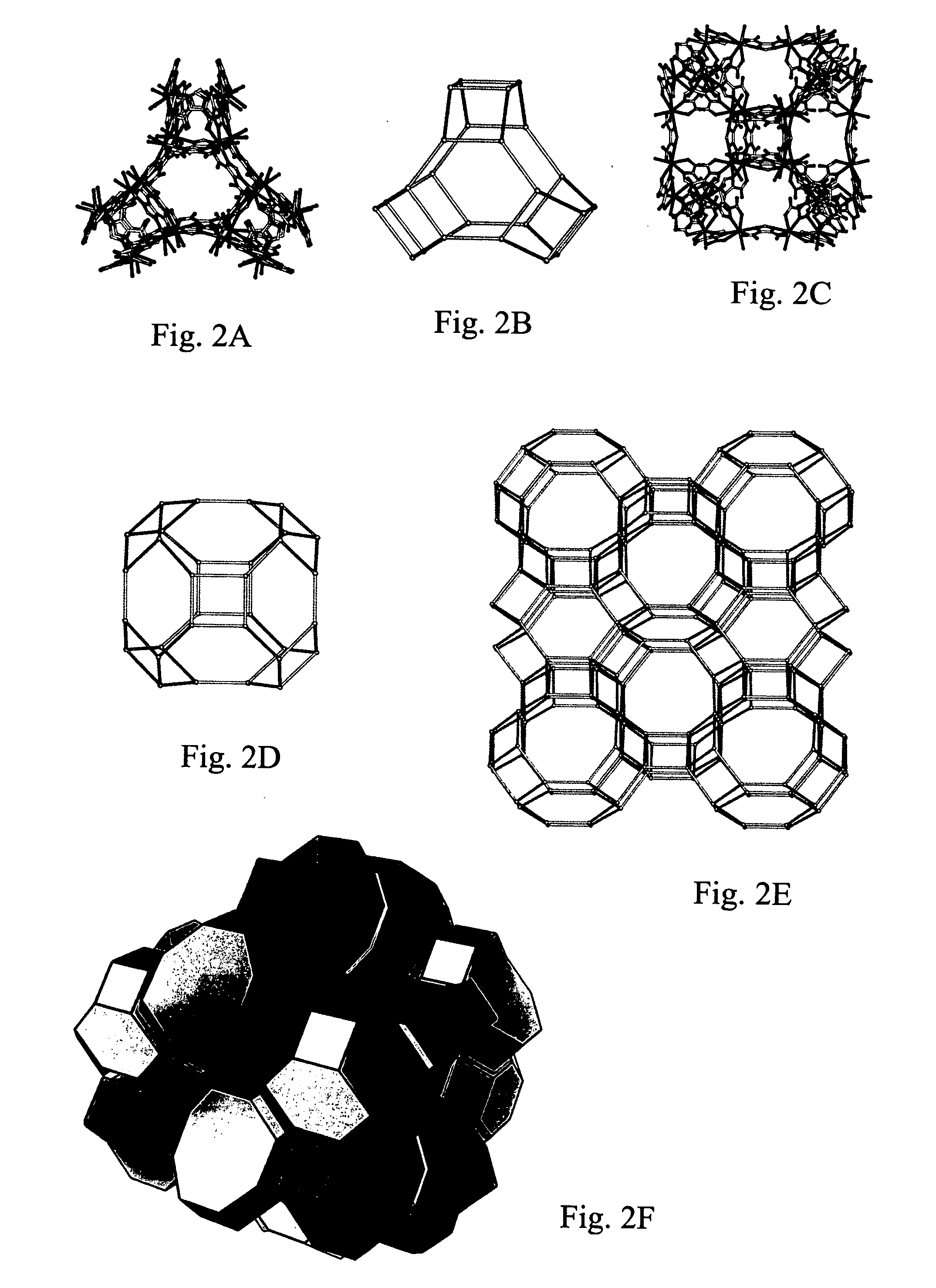
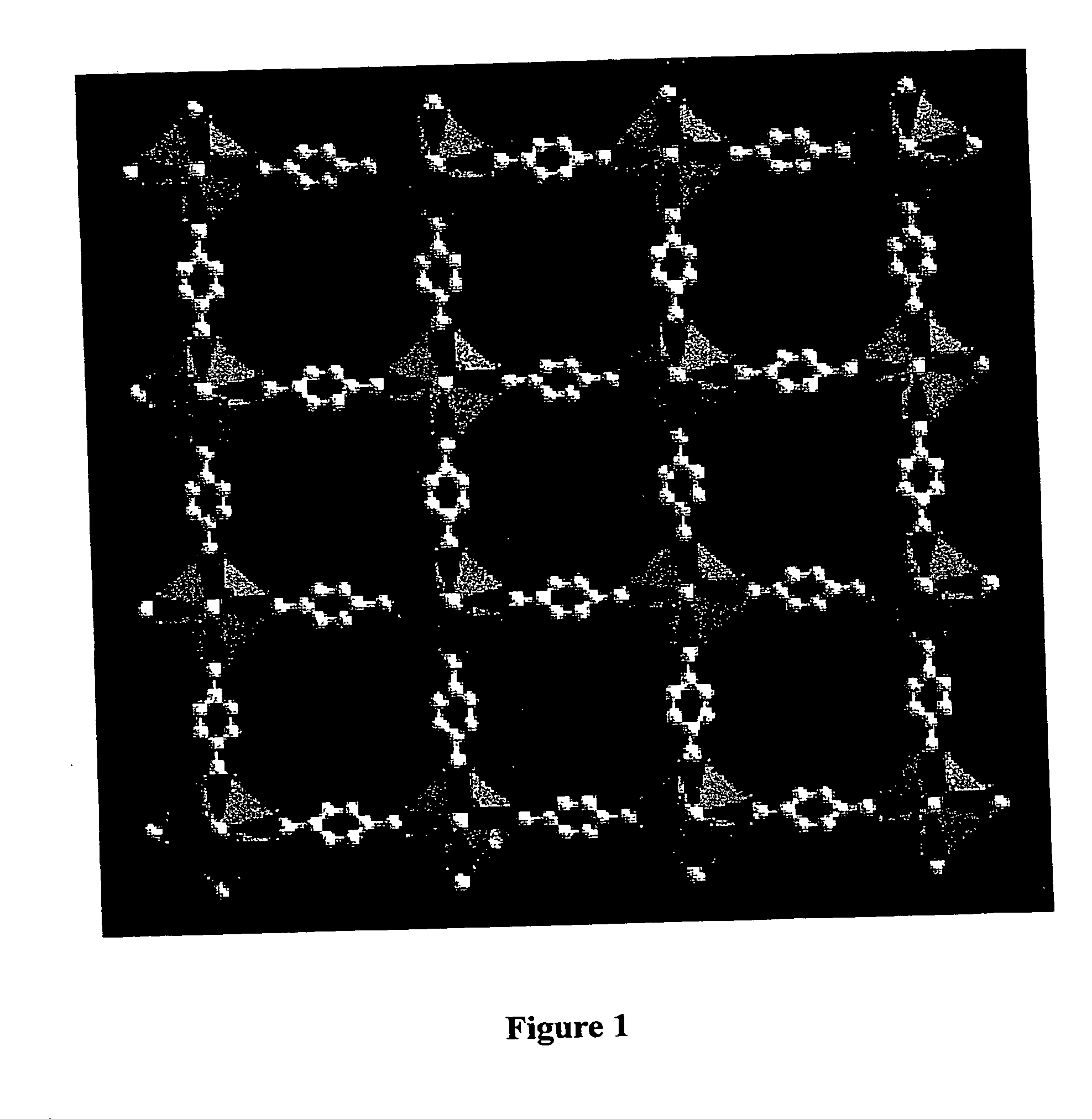
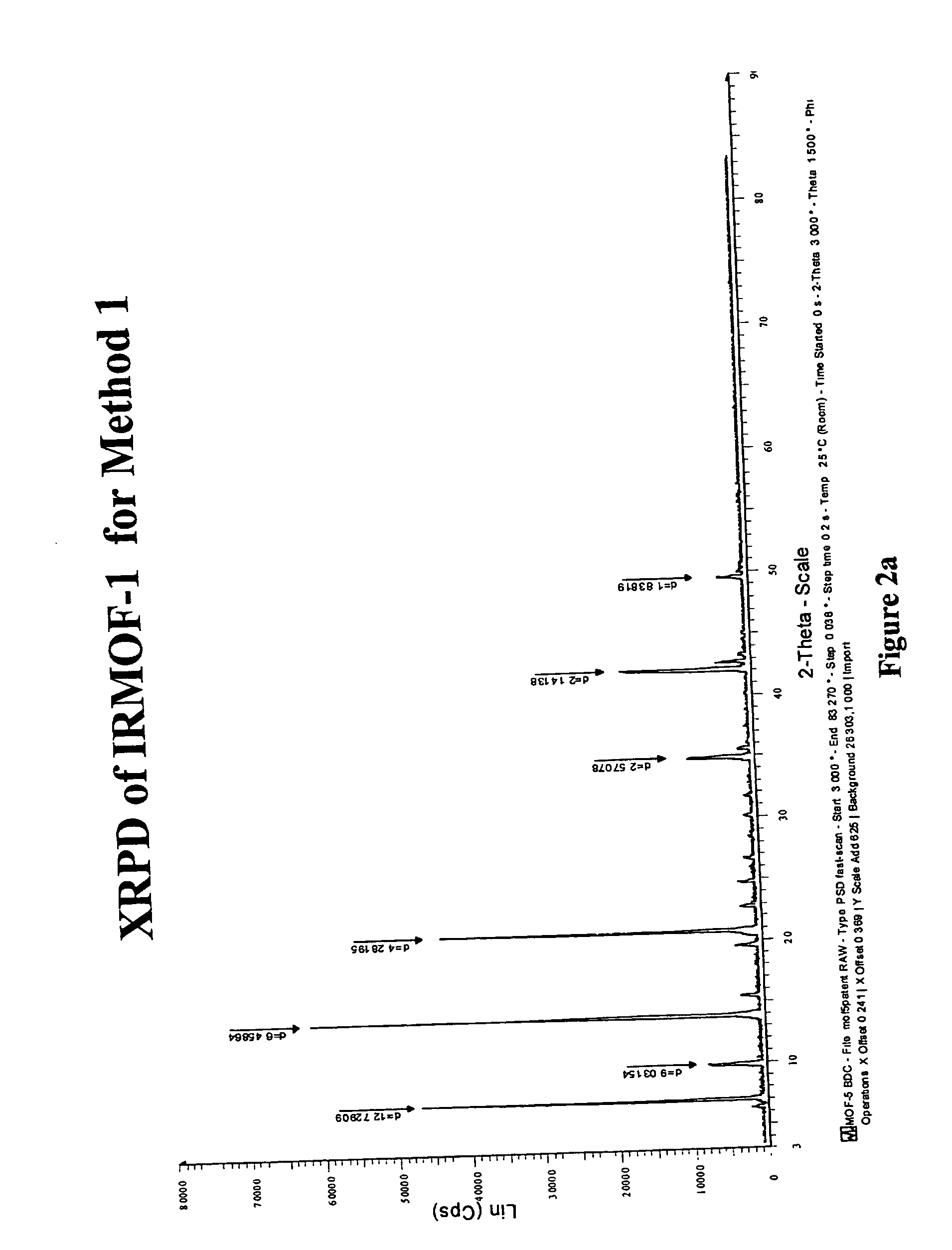
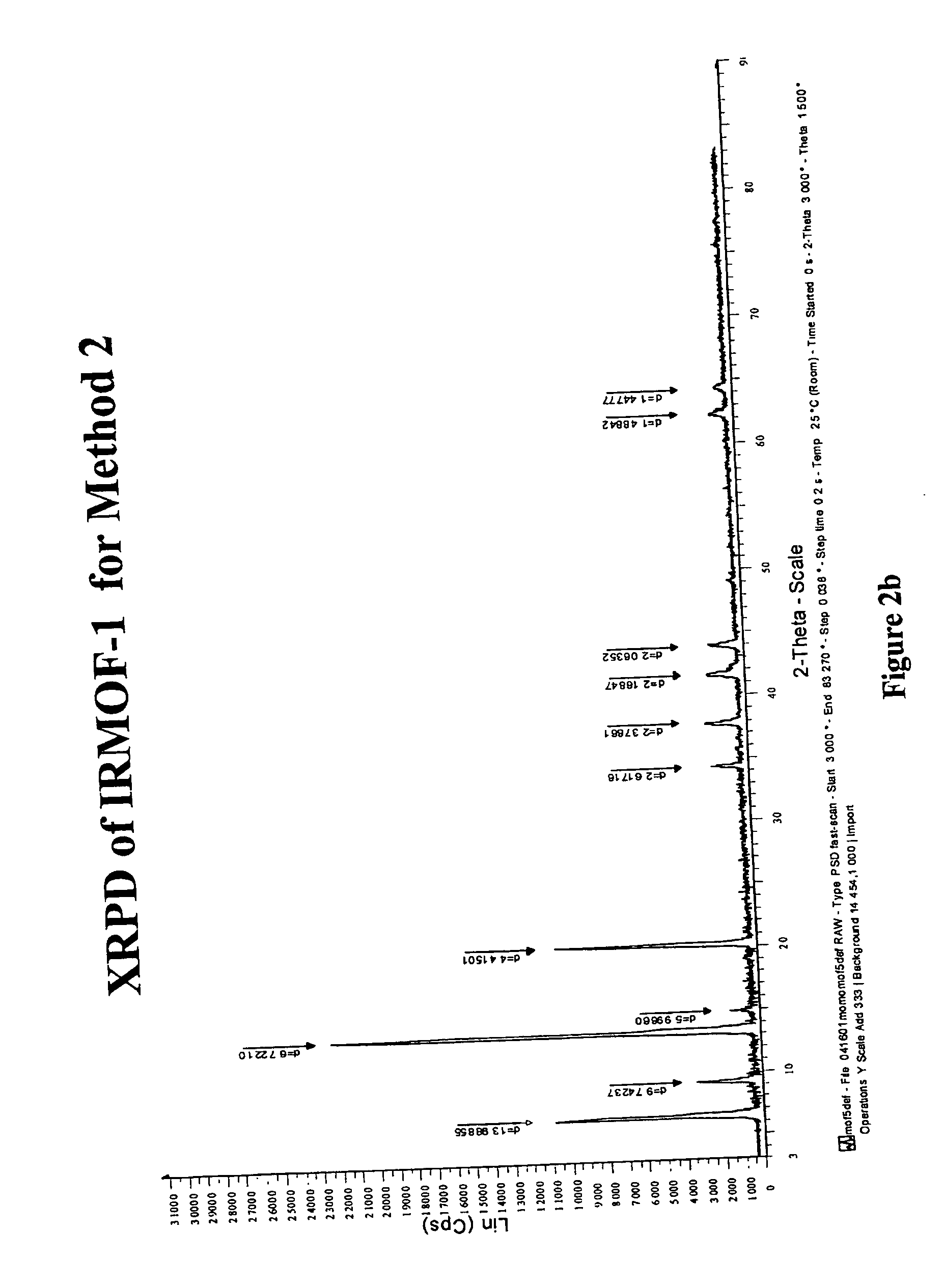
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS6930193B2High methane storage capacityIncrease storage capacityGroup 5/15 element organic compoundsGroup 8/9/10/18 element organic compoundsOrganic linkingSystems design
An isoreticular metal-organic framework (IRMOF) and method for systematically forming the same. The method comprises the steps of dissolving at least one source of metal cations and at least one organic linking compound in a solvent to form a solution; and crystallizing the solution under predetermined conditions to form a predetermined IRMOF. At least one of functionality, dimension, pore size and free volume of the IRMOF is substantially determined by the organic linking compound.
Owner:RGT UNIV OF MICHIGAN
Films having a desiccant material incorporated therein and methods of use and manufacture
InactiveUS20070160789A1Improve efficacyExtended shelf lifeSurgical furnitureDiagnosticsDesiccantInsertion stent
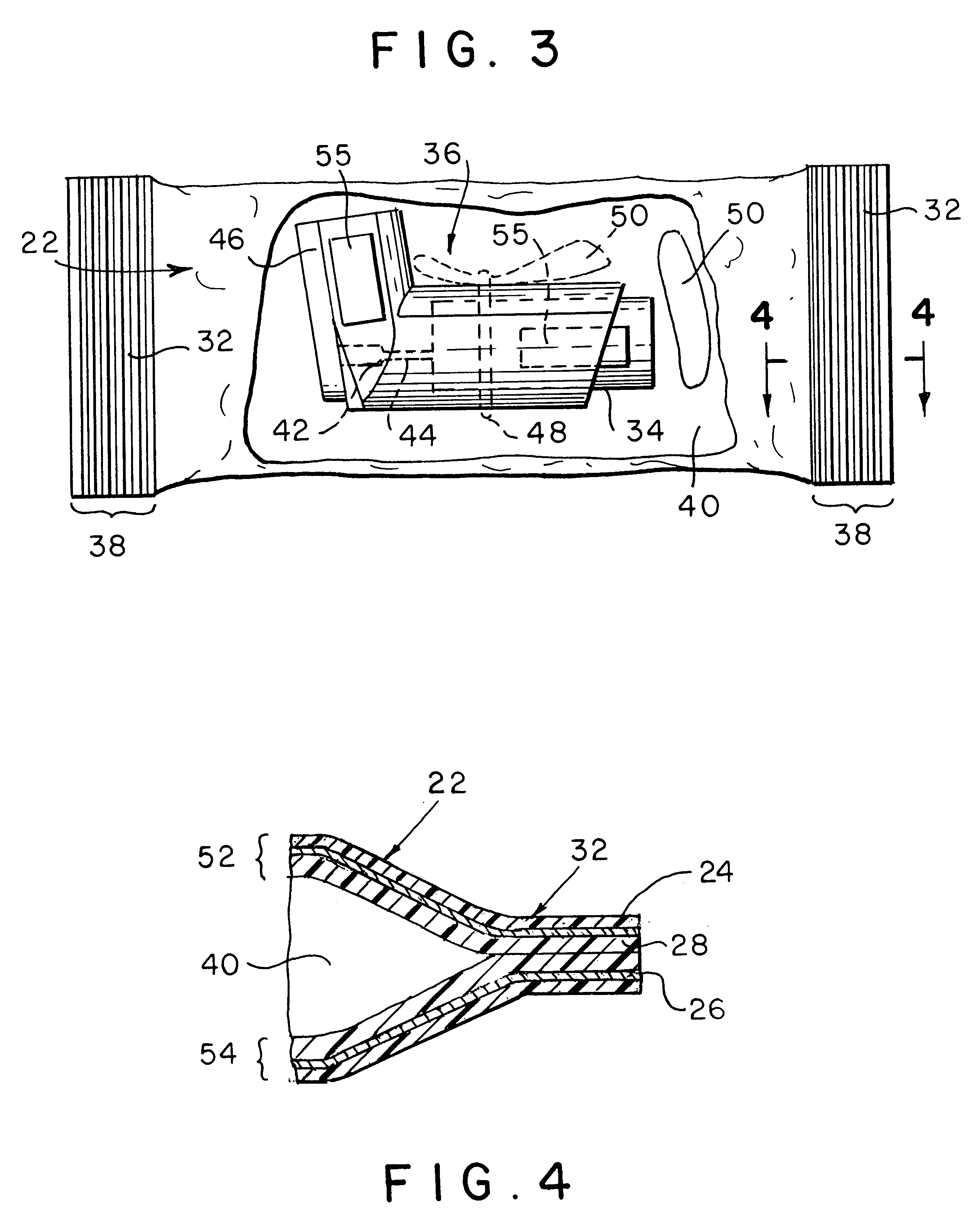
Film structures, packages, films and methods of making the same are provided wherein the film structures have a desiccant material incorporated into at least one layer of the film structures and further wherein the film structures can comprise a material for making a peelable seal when the film structures are heat sealed to other film structures. The film structures are utilized for a package to hold a product that may be sensitive to the presence of moisture. The product may preferably be pharmaceutical products, nutraceutical products, or devices such as absorbable sutures or medical stents, although any moisture-sensitive product is contemplated by the present invention.
Owner:ALCAN PACKAGING FLEXIBLE FRANCE
Method and package for storing a pressurized container containing a drug
InactiveUS6179118B1Reduce manufacturing complexityReduce manufacturing costWrappersDiagnosticsWater vaporWaste management
Owner:GLAXO SMITHKLINE LLC
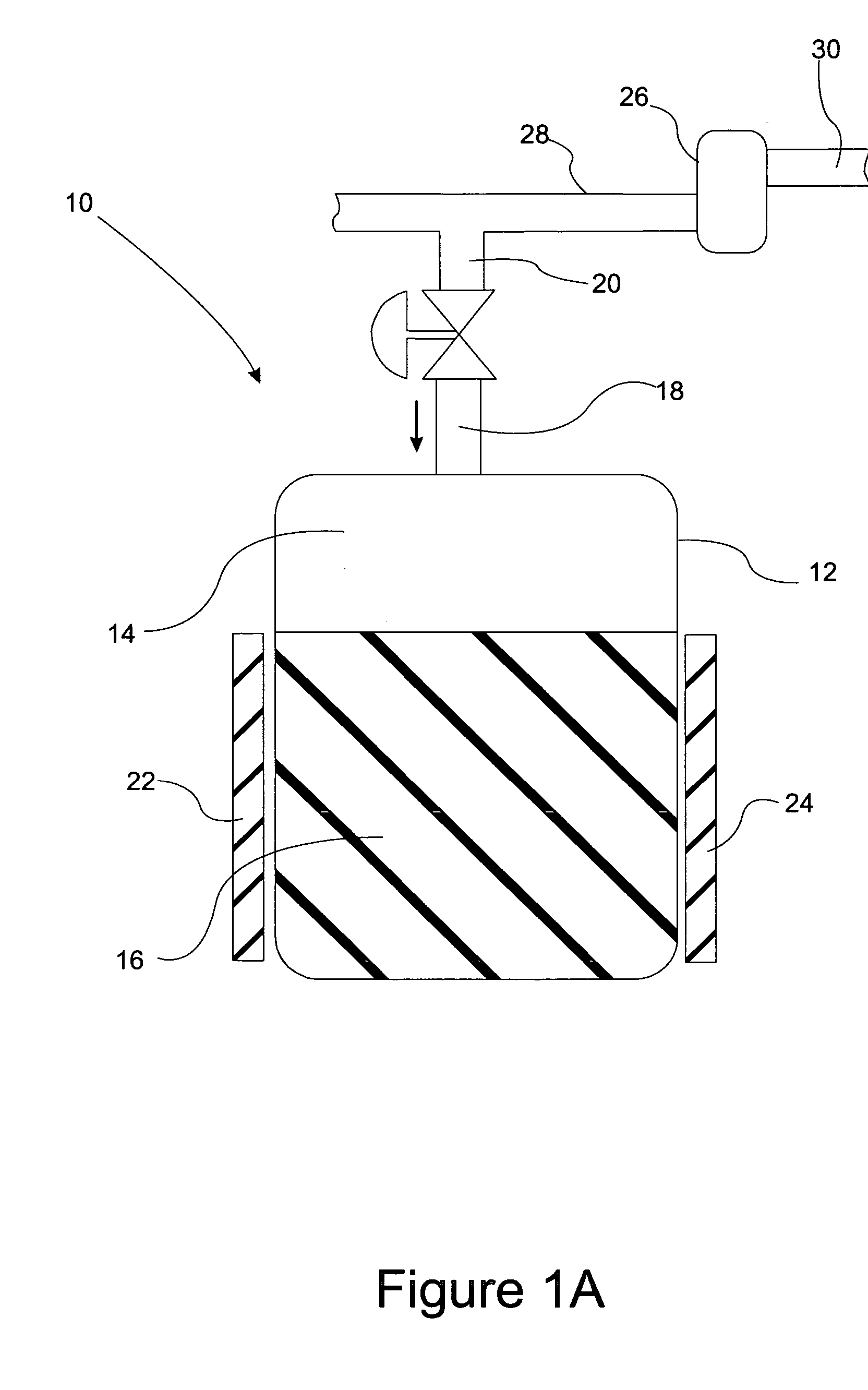
Sorbent-based gas storage and delivery system for dispensing of high-purity gas, and apparatus and process for manufacturing semiconductor devices, products and precursor structures utilizing same
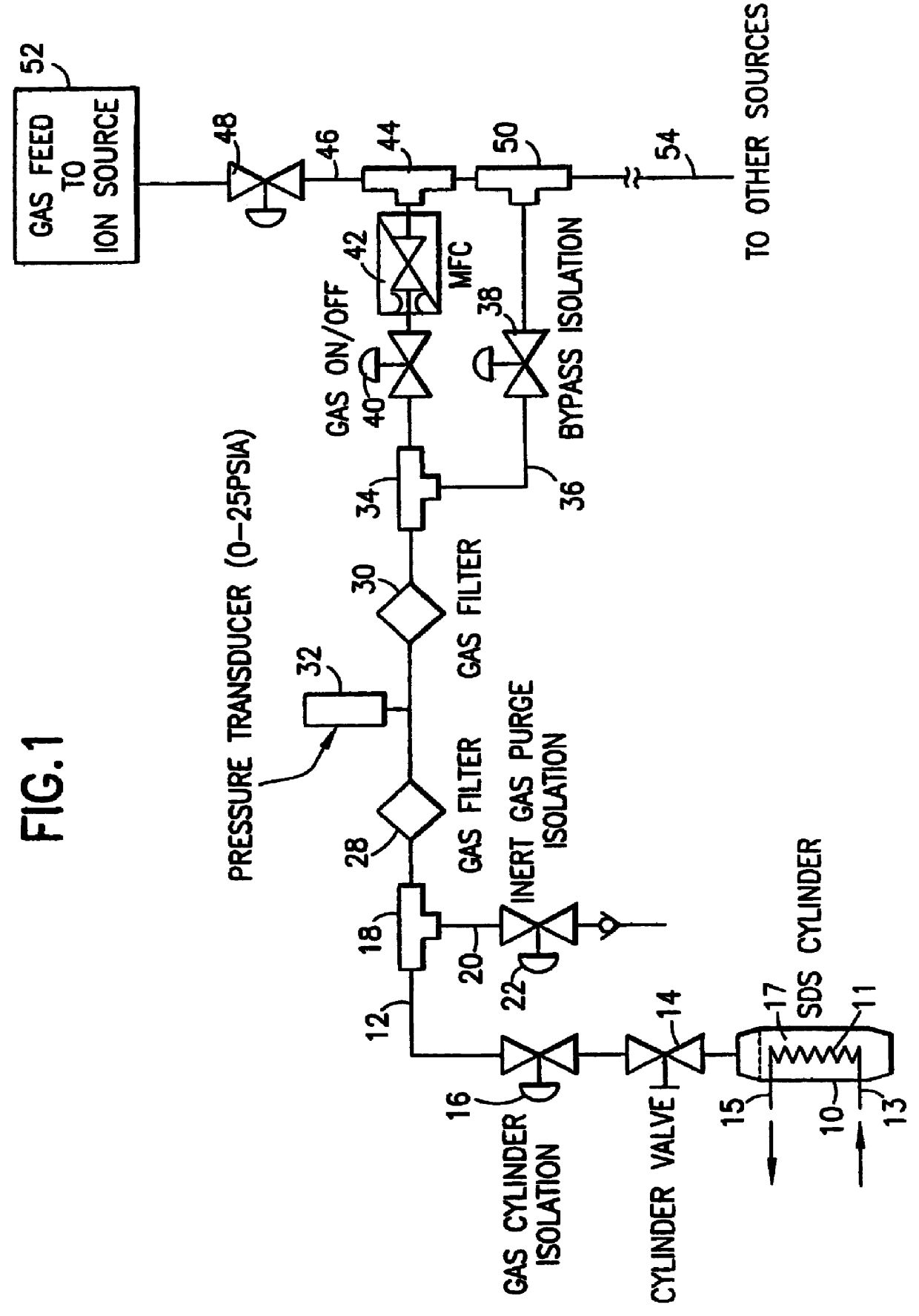
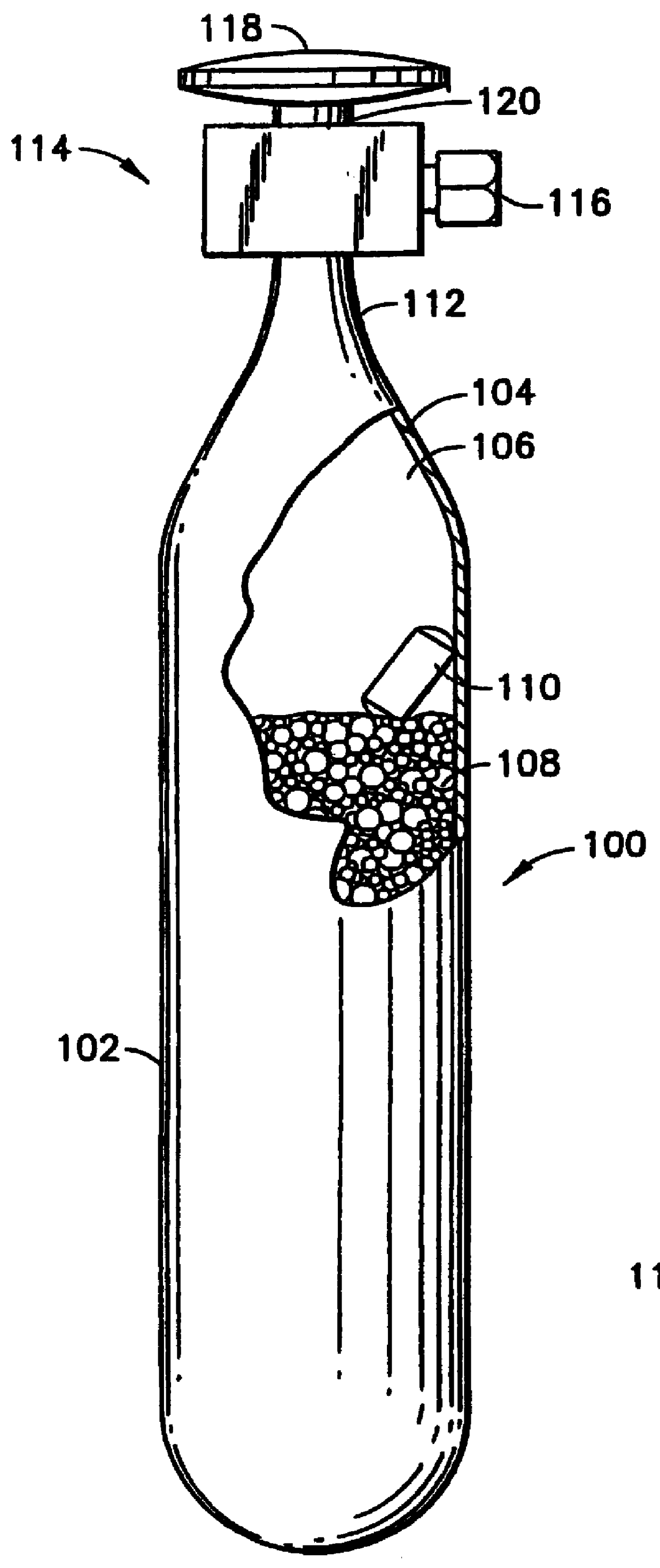
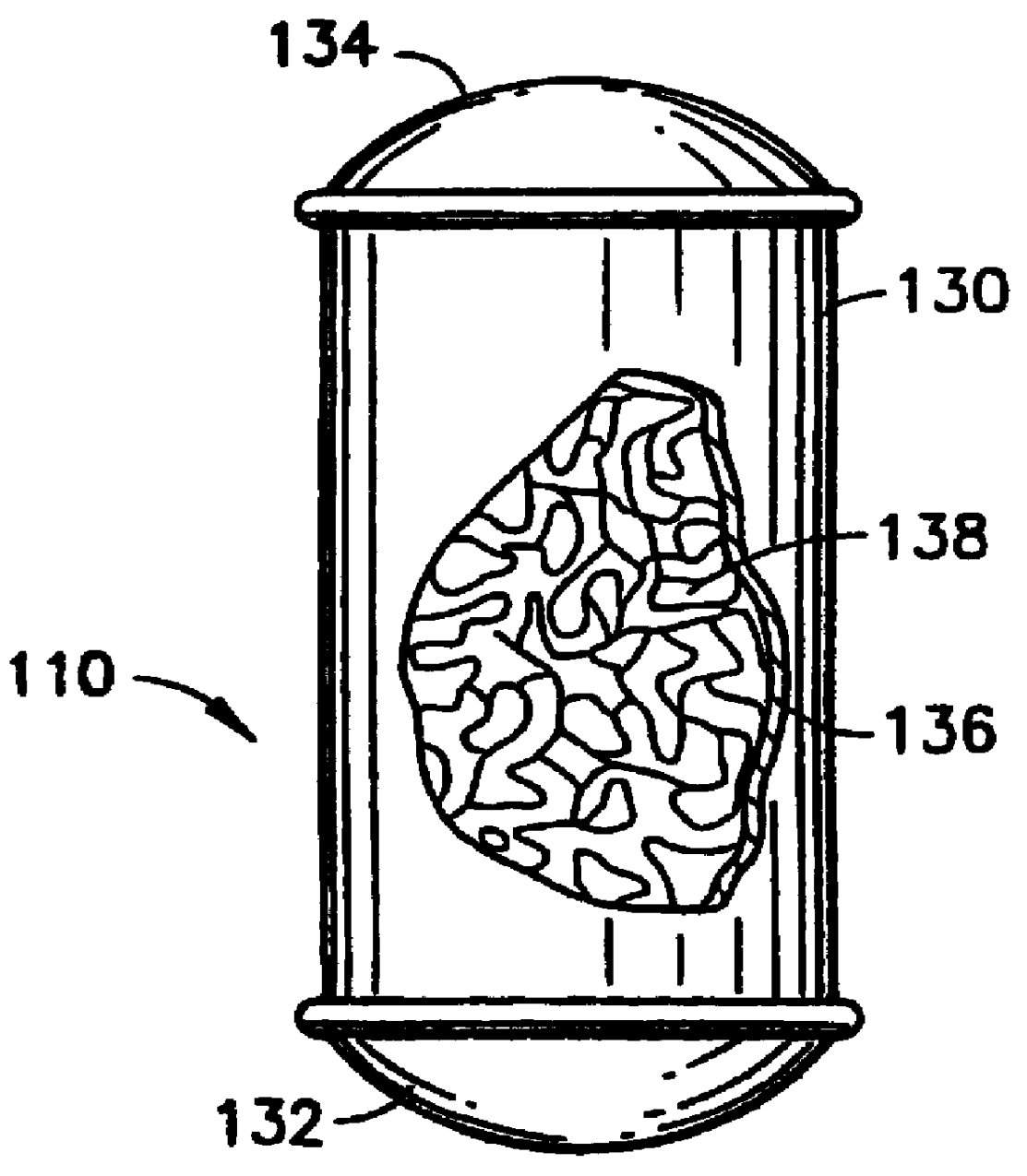
A sorbent-based gas storage and dispensing system, including a storage and dispensing vessel containing a solid-phase physical sorbent medium having a sorbate gas physically adsorbed thereon. A chemisorbent material is provided in the vessel to chemisorb the impurities for gas phase removal thereof in the storage and dispensing vessel. Desorbed sorbate gas is discharged from the storage and dispensing vessel by a dispensing assembly coupled to the vessel. The chemisorbent may be provided in a capsule including an impurity-permeable, but sorbate gas-impermeable membrane, and installed in the vessel at the time of sorbent material loading. Semiconductor manufacturing processes and products manufactured by such processes are described.
Owner:ENTEGRIS INC
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS7196210B2Group 8/9/10/18 element organic compoundsGroup 5/15 element organic compoundsSystems designMetal-organic framework
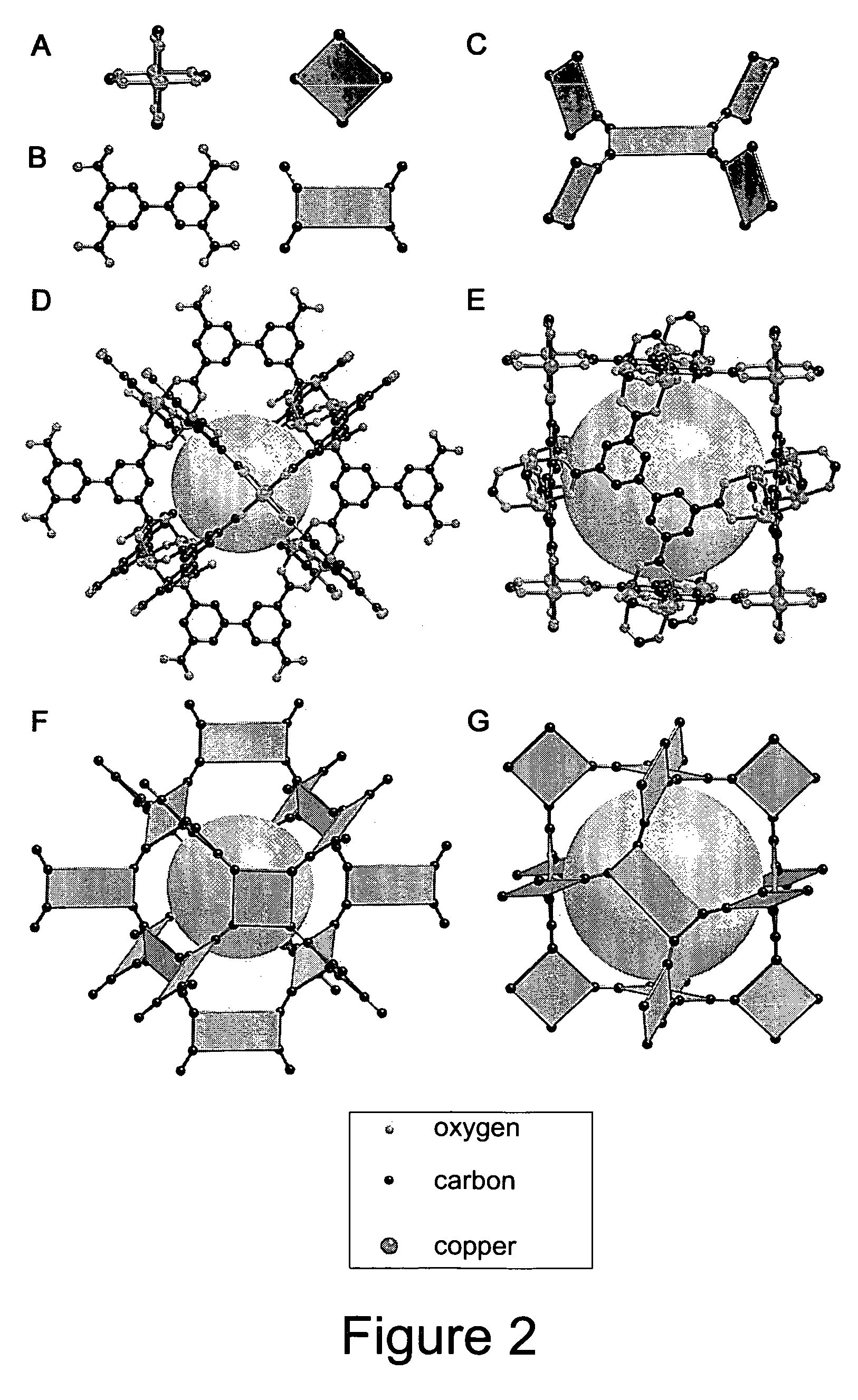
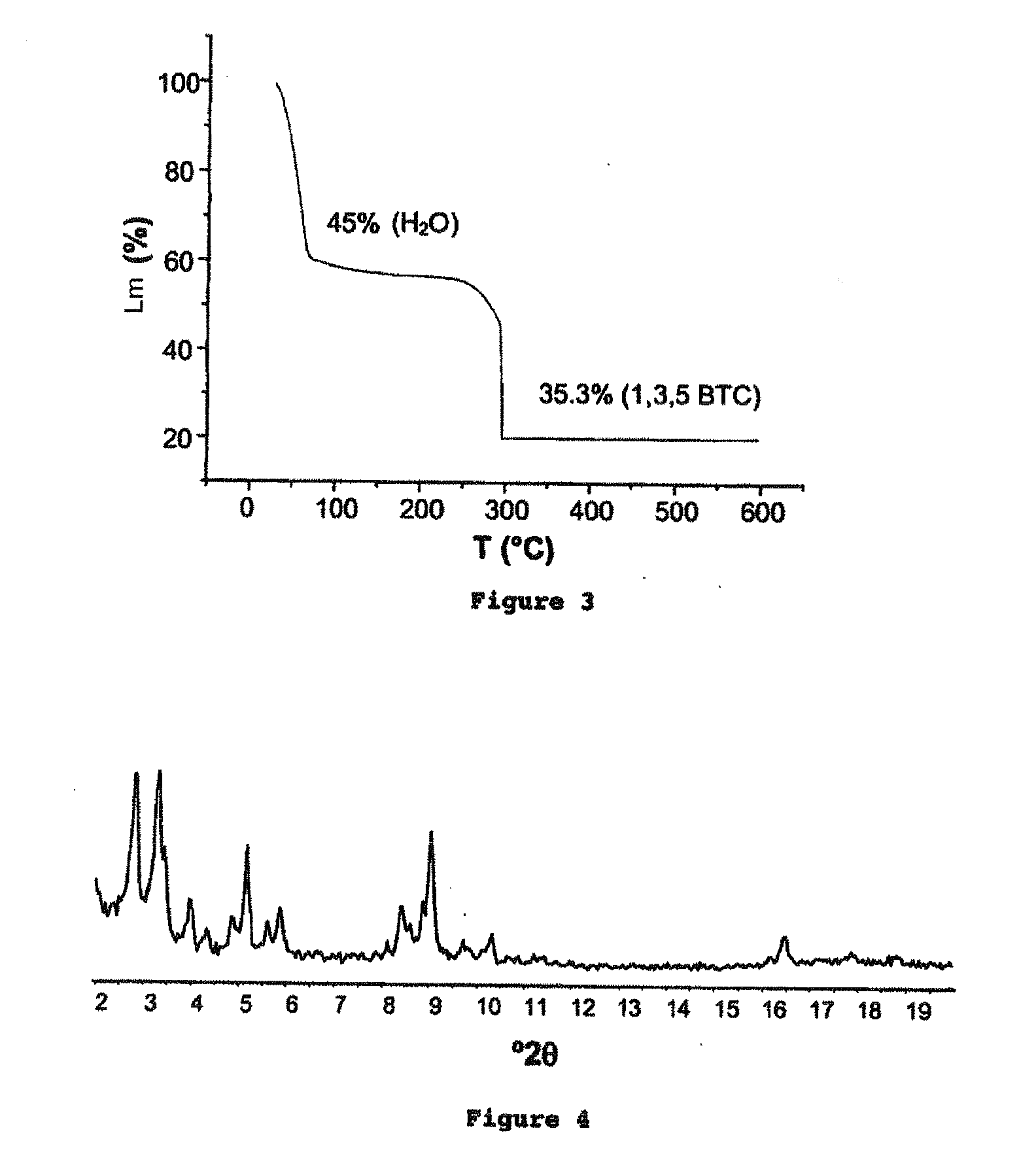
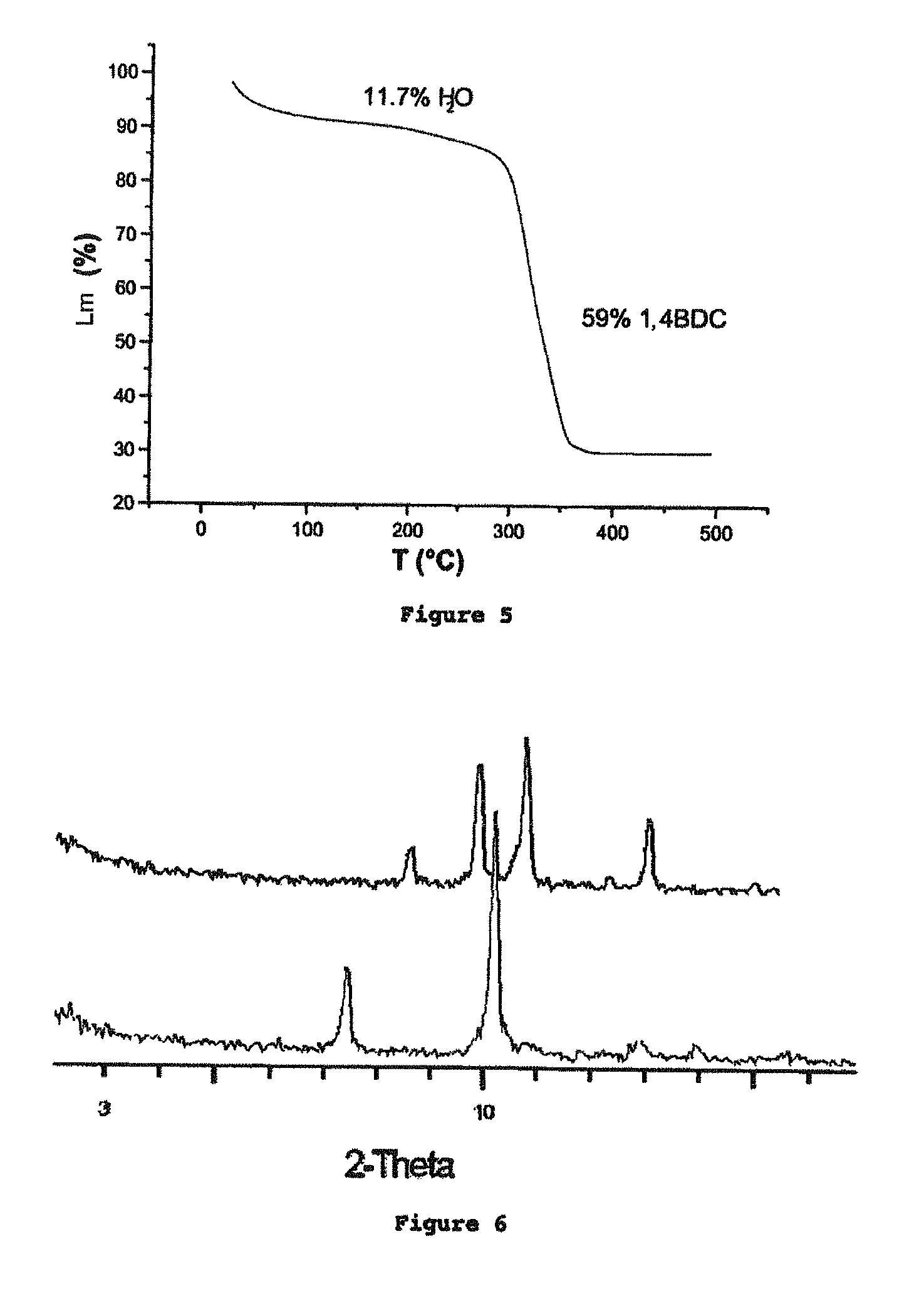
The ability to design and construct solid-state materials with pre-determined structures is a grand challenge in chemistry. An inventive strategy based on reticulating metal ions and organic carboxylate links into extended networks has been advanced to a point that has allowed the design of porous structures in which pore size and functionality can be varied systematically. MOF-5, a prototype of a new class of porous materials and one that is constructed from octahedral Zn—O—C clusters and benzene links, was used to demonstrate that its 3-D porous system can be functionalized with the organic groups, —Br, —NH2, —OC3H7, —OC5H11, —H4C2, and —H4C4, and its pore size expanded with the long molecular struts biphenyl, tetrahydropyrene, pyrene, and terphenyl. The ability to direct the formation of the octahedral clusters in the presence of a desired carboxylate link is an essential feature of this strategy, which resulted in the design of an isoreticular (having the same framework topology) series of sixteen well-defined materials whose crystals have open space representing up to 91.1% of the crystal volume, and homogeneous periodic pores that can be incrementally varied from 3.8 to 28.8 angstroms. Unlike the unpredictable nature of zeolite and other molecular sieve syntheses, the deliberate control exercised at the molecular level in the design of these crystals is expected to have tremendous implications on materials properties and future technologies. Indeed, data indicate that members of this series represent the first monocrystalline mesoporous organic / inorganic frameworks, and exhibit the highest capacity for methane storage (155 cm3 / cm3 at 36 atm) and the lowest densities (0.41 to 0.21 g / cm3) attained to date for any crystalline material at room temperature.
Owner:RGT UNIV OF MICHIGAN
High gas adsorption in a microporous metal-organic framework with open-metal sites
A gas storage material contains a metal-organic framework that includes a plurality of metal clusters and a plurality of charged multidentate linking ligands that connect adjacent metal clusters. Each metal cluster includes one or more metal ions and at least one open metal site. The metal-organic framework includes one or more sites for storing molecular hydrogen. A hydrogen storage system using the hydrogen storage material is provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
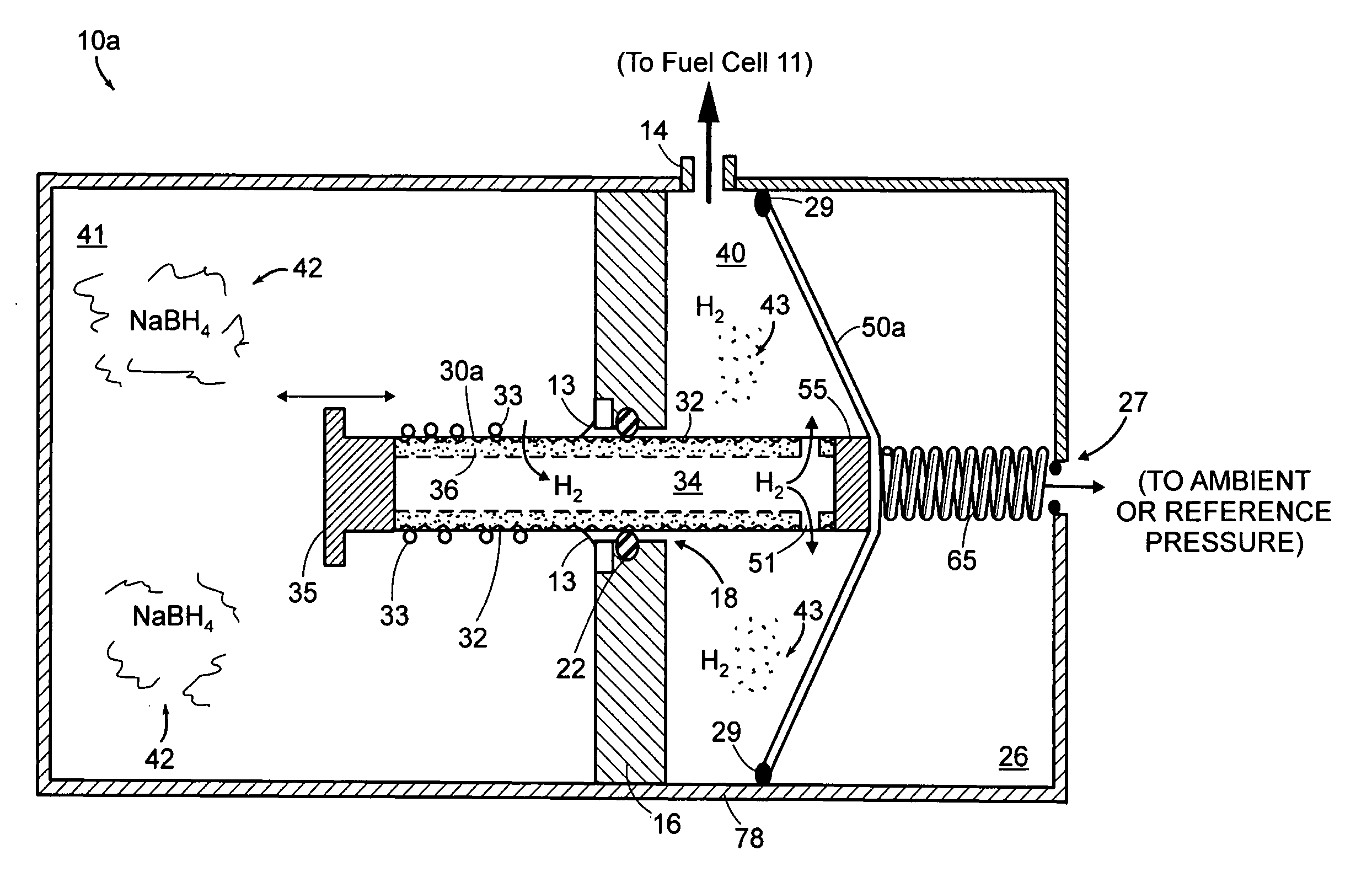
Self-regulating gas generator and method
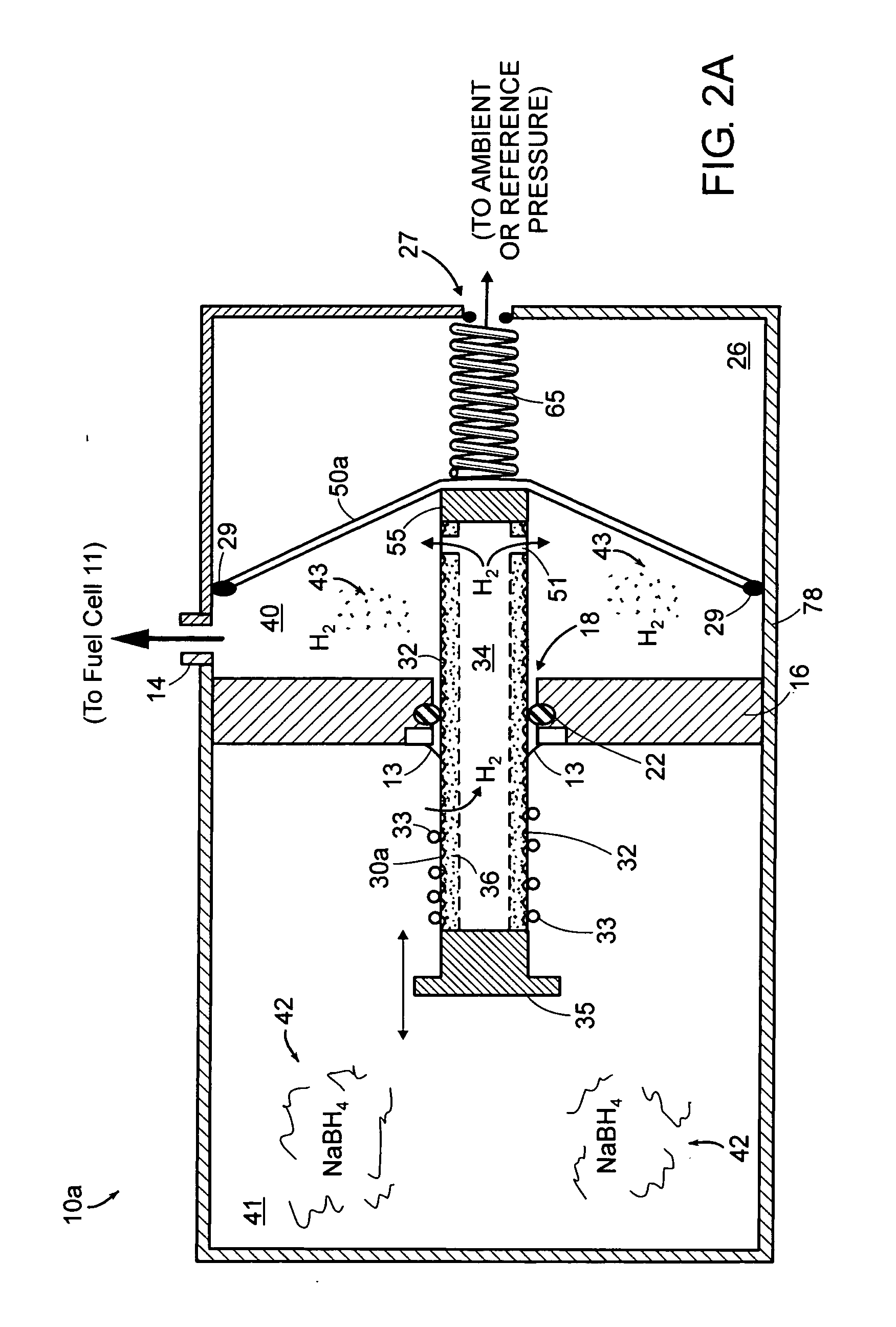
ActiveUS20050158595A1Increase and decreases gas production rateExtend your lifeReactant parameters controlMultiple metal hydridesHydrogenFuel cells
A self-regulating gas generator that, in response to gas demand, supplies and automatically adjusts the amount of gas (e.g., hydrogen or oxygen) catalytically generated in a chemical supply chamber from an appropriate chemical supply, such as a chemical solution, gas dissolved in liquid, or mixture. The gas generator may employ a piston, rotating rod, or other element(s) to expose the chemical supply to the catalyst in controlled amounts. The gas generator may be used to provide gas for various gas consuming devices, such as a fuel cell, torch, or oxygen respiratory devices.
Owner:ENCITE LLC
Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room-temperature
InactiveUS20070068389A1Promote absorptionSufficiently flexibleInternal combustion piston enginesExhaust apparatusCo2 storageRoom temperature
A carbon dioxide storage system includes a container and a conduit attached to the container for introducing or removing a carbon dioxide-containing composition from the container. A carbon dioxide storage material is positioned within the container. The carbon dioxide-storage material includes a metal-organic framework, which has a sufficient surface area to store at least 10 carbon dioxide molecules per formula unit of the metal-organic framework at a temperature of about 25° C.
Owner:RGT UNIV OF MICHIGAN
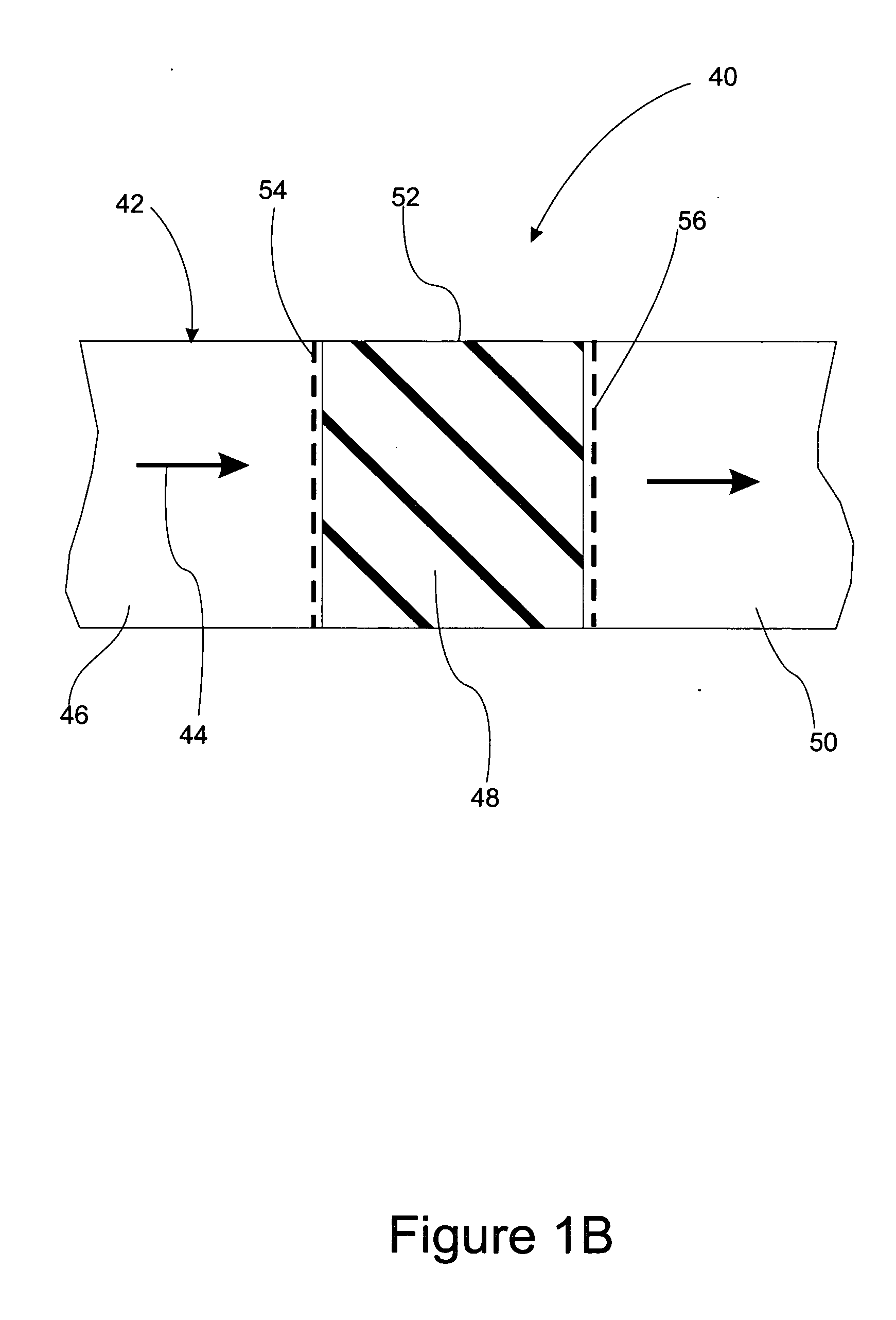
Fuel supply for a fuel cell
InactiveUS6924054B2Lower the volumeIncrease volumeInternal combustion piston enginesReactant parameters controlFuel cellsFuel supply
The present invention concerns a fuel supply for a fuel cell. The fuel supply includes a fuel storage area configured to hold a fuel solution, a fuel solution outlet configured to pass the fuel solution from the fuel storage area, a waste storage area, a waste inlet configured to pass waste into the waste storage area, and a movable barrier separating the fuel storage area and the waste storage area. The movable barrier is configured to move as fuel solution is passed from the fuel storage area and waste solution is passed into the waste storage area to simultaneously decrease the volume of the fuel storage area and increase the volume of the waste storage area.
Owner:INTELLIGENT ENERGY LTD
Array of packages having relative size indicators
An array of packages wherein each package has relative size indicators is provided. The relative size indicators can be any indicator suitable for visually communicating to a consumer, the relative size of the absorbent articles contained in a package, in relation to other absorbent articles contained in other packages within an array of packages. The packages can comprise an interior surface and an exterior surface, the interior surface defining an interior space. Absorbent articles can be disposed within the interior space and the exterior surface can include relative size indicators.
Owner:THE PROCTER & GAMBLE COMPANY
Power plant with energy recovery from fuel storage
InactiveUS20020112479A1Fuel cell heat exchangeInternal combustion piston enginesWorking fluidPower station
Power plant systems and processes are described that enable recovery of at least a portion of the fuel storage energy associated with a storage system for supplying fuel to the power plant systems. A first embodiment of an energy-recovery power plant system includes at least one fuel storage container and at least one expander that can receive fuel from the fuel storage container at a first pressure and provide the fuel to the power plant at a second pressure that is lower than the first pressure. A second embodiment of an energy-recovery power plant system includes a first conduit fluidly coupling the fuel storage container and the power plant for delivering fuel from the fuel storage container to the power plant and at least one regenerative thermodynamic cycle engine thermally coupled to the first conduit such that heat may be exchanged between the fuel and a working fluid for the regenerative thermodynamic cycle engine.
Owner:QUSIR TECH
Method and apparatus for producing ammonia (NH3)
InactiveUS6928807B2High activityNitrogen compoundsInternal combustion piston enginesHydrolysisRapid thermal processing
A method is provided for producing ammonia (NH3) and introducing the produced ammonia (NH3) into an exhaust gas stream as a reduction means for selectively catalytically reducing nitrogen oxides contained in the exhaust gas stream, which is an exhaust stream generated by the combustion process of a motor, a gas turbine, or a burner. The method comprises feeding dry urea from a supply container in a controlled amount to reactor and subjecting the dry urea in the reactor to a sufficiently rapid thermal treatment such that a gas mixture comprising the reaction products of ammonia (NH3) and isocyanic acid (HCNO) is created. Also, the method comprises immediately catalytically treating the thus produced gas mixture in the presence of water such that the isocyanic acid (HCNO) resulting from the rapid thermal treatment is converted, via quantitative hydrolysis treatment, into ammonia (NH3) and carbon dioxide (CO2).
Owner:MAN NUTZFAHRZEUGE AG
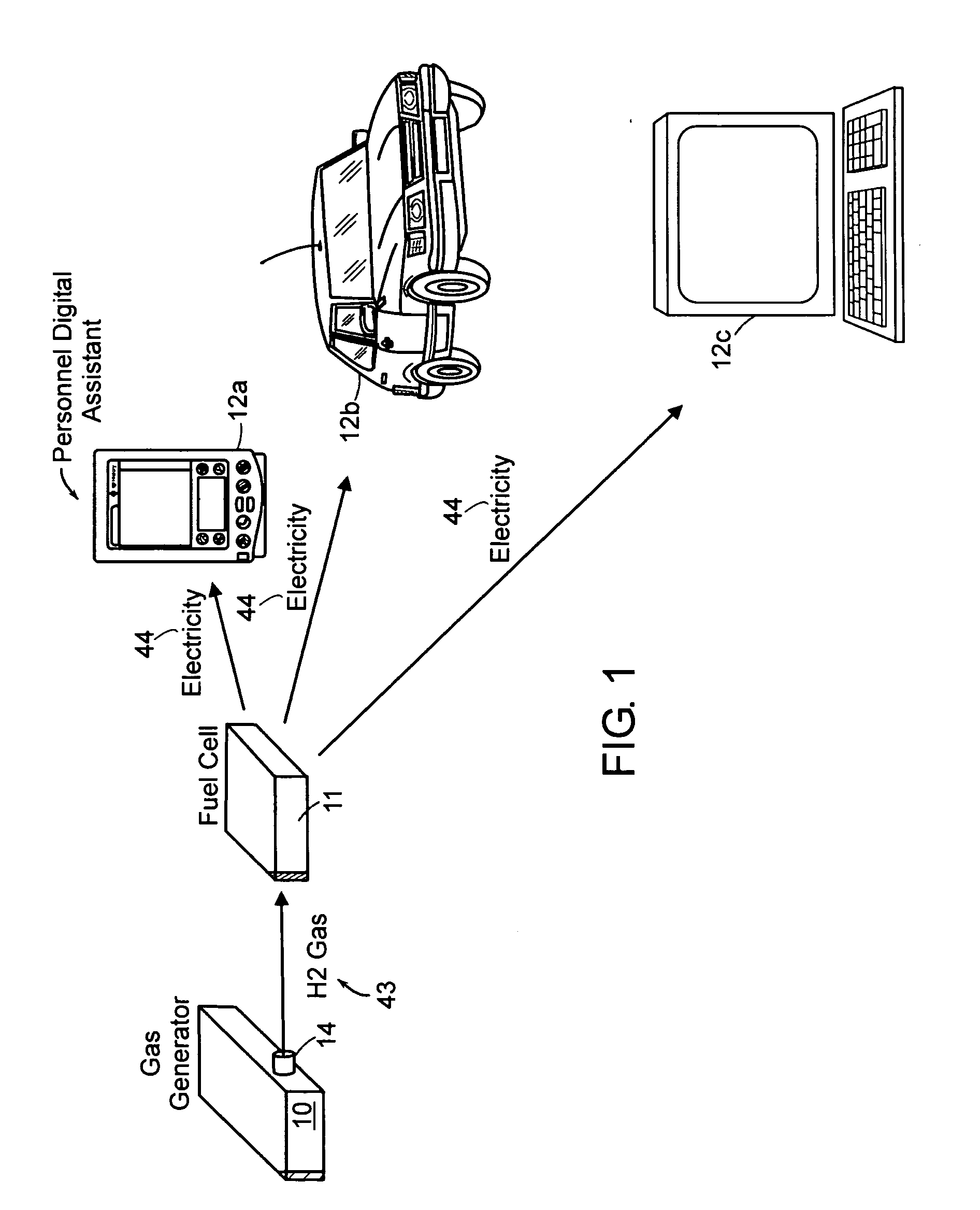
Portable hydrogen generator
A hydrogen generation system includes a fuel container, a spent fuel container, a catalyst system and a control system for generating hydrogen in a manner which provides for a compact and efficient construction while producing hydrogen from a reaction involving a hydride solution such as sodium borohydride.
Owner:SILICON VALLEY BANK
High gas adsorption metal-organic framework
A gas storage material contains a metal-organic framework that includes a plurality of metal clusters and a plurality of charged multidentate linking ligands that connect adjacent metal clusters. Each metal cluster includes one or more metal ions and at least one open metal site. The metal-organic framework includes one or more sites for storing molecular hydrogen. A hydrogen storage system using the hydrogen storage material is provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Activated carbon cryogels and related methods
Owner:BASF SE +1
Finely divided metal catalyst and method for making same
InactiveUS6841512B1Facilitating hydrogen consumptionSmall particle sizeReactant parameters controlReversible hydrogen uptakeParticulatesMetal catalyst
An inexpensive, highly catalytic material preferably formed by a leaching process. The catalyst comprises a finely divided metal particulate and a support. The active material may be a nickel and / or nickel nickel alloy particulate having a particle size less than about 100 Angstroms. The support may be one or more metal oxides.
Owner:CHEVRONTEXACO TECH VENTURES
Sorbent-based fluid storage and dispensing vessel with replaceable sorbent cartridge members
InactiveUS6019823AQuick degassingQuickly and easily and separately preparedGas treatmentOther chemical processesSorbentProduct gas
Solid-phase physical sorbent medium holding adsorbed fluid is provided in a cartridge, for use in a sorbent-based fluid storage and dispensing system. One or more of such cartridges may be disposed in a fluid storage and dispensing vessel and opened prior or subsequent to sealing of the vessel, to provide desorbable fluid for dispensing from the vessel, e.g., by pressure differential, concentration differential and / or thermal desorption. Use of such cartridges thereby obviates the sorbent bake-out and sorbate gas loading steps necessary in prior practice, thereby simplifying the manufacture of the fluid storage and dispensing system.
Owner:ENTEGRIS INC
Zeolite-like metal organic frameworks (ZMOFS): modular approach to the synthesis of organic-inorganic hybrid porous materials having a zeolite like topology
InactiveUS20060287190A1Molecular sieve catalystsOther chemical processesMetal-organic frameworkModularity
The subject invention pertains to metal organic frameworks (MOF) having zeolite-net-like topology, their methods of use, and their modes of synthesis. The ZMOFs are produced by combining predesigned tetrahedral building, generated in situ using heterochelation, with polyfunctional ligands that have the commensurate angle and the required donor groups for the chelation. Each molecular building block is contrasted of a single metal ion and ligands with both heterochelation functionality and bridging functionality. Advantageously, zeolite-net-like MOFs of the subject invention are porous and contain large functional cavities, which is useful for encapsulating large molecules.
Owner:UNIV OF SOUTH FLORIDA
Systems and methods for forming metal oxides using alcohols
A method of forming (and an apparatus for forming) a metal oxide layer on a substrate, particularly a semiconductor substrate or substrate assembly, using a vapor deposition process, one or more alcohols, and one or more metal-containing precursor compounds.
Owner:MICRON TECH INC
Clathrate hydrate modular storage, applications and utilization processes
Methods, apparatuses and systems directed to clathrate hydrate modular storage, applications and utilization processes. In one implementation, the present invention provides a method of creating scalable, easily deployable storage of natural gas and thermal energy by assembling an array of interconnecting, modular gas clathrate hydrate storage units.
Owner:SOLID GAS TECH
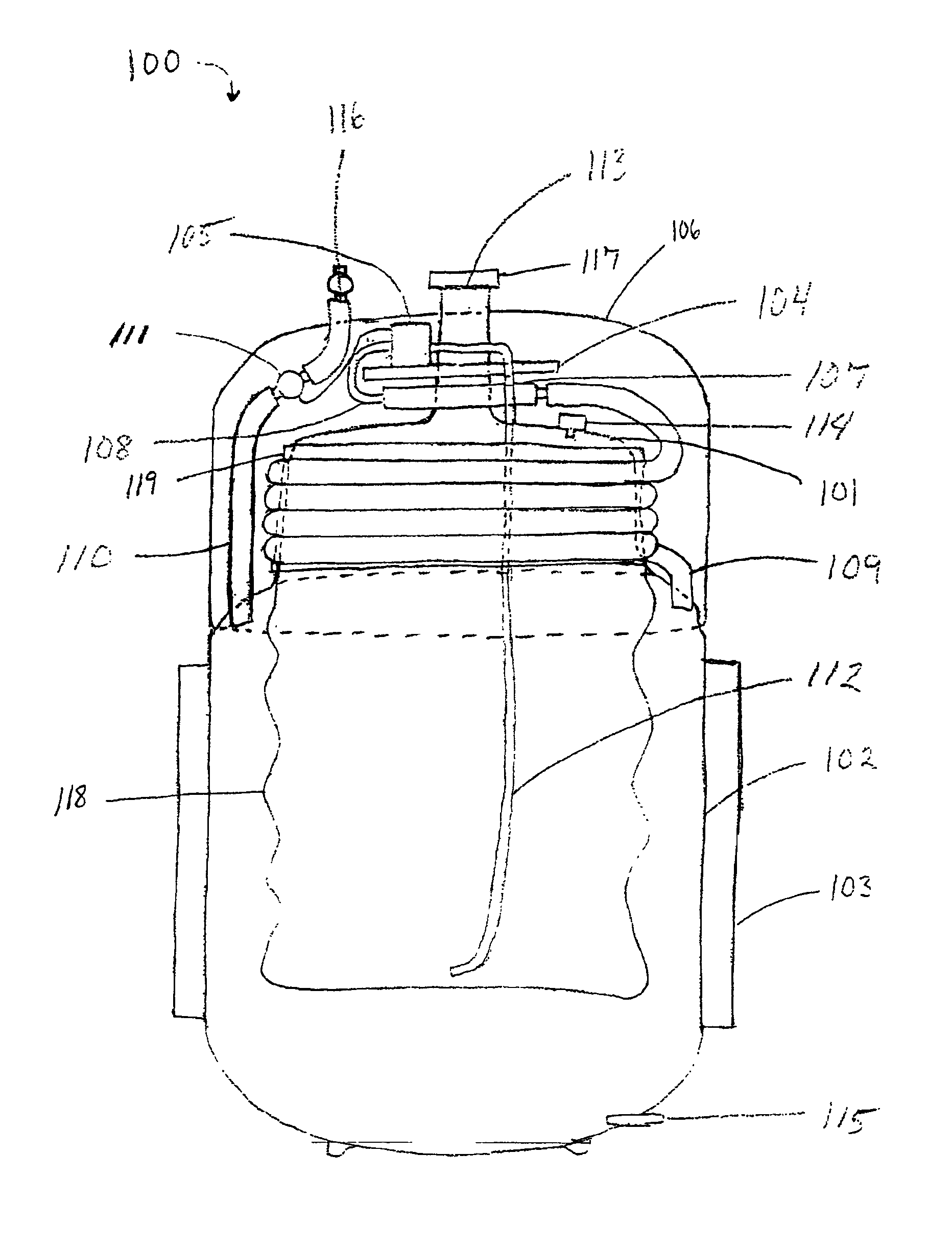
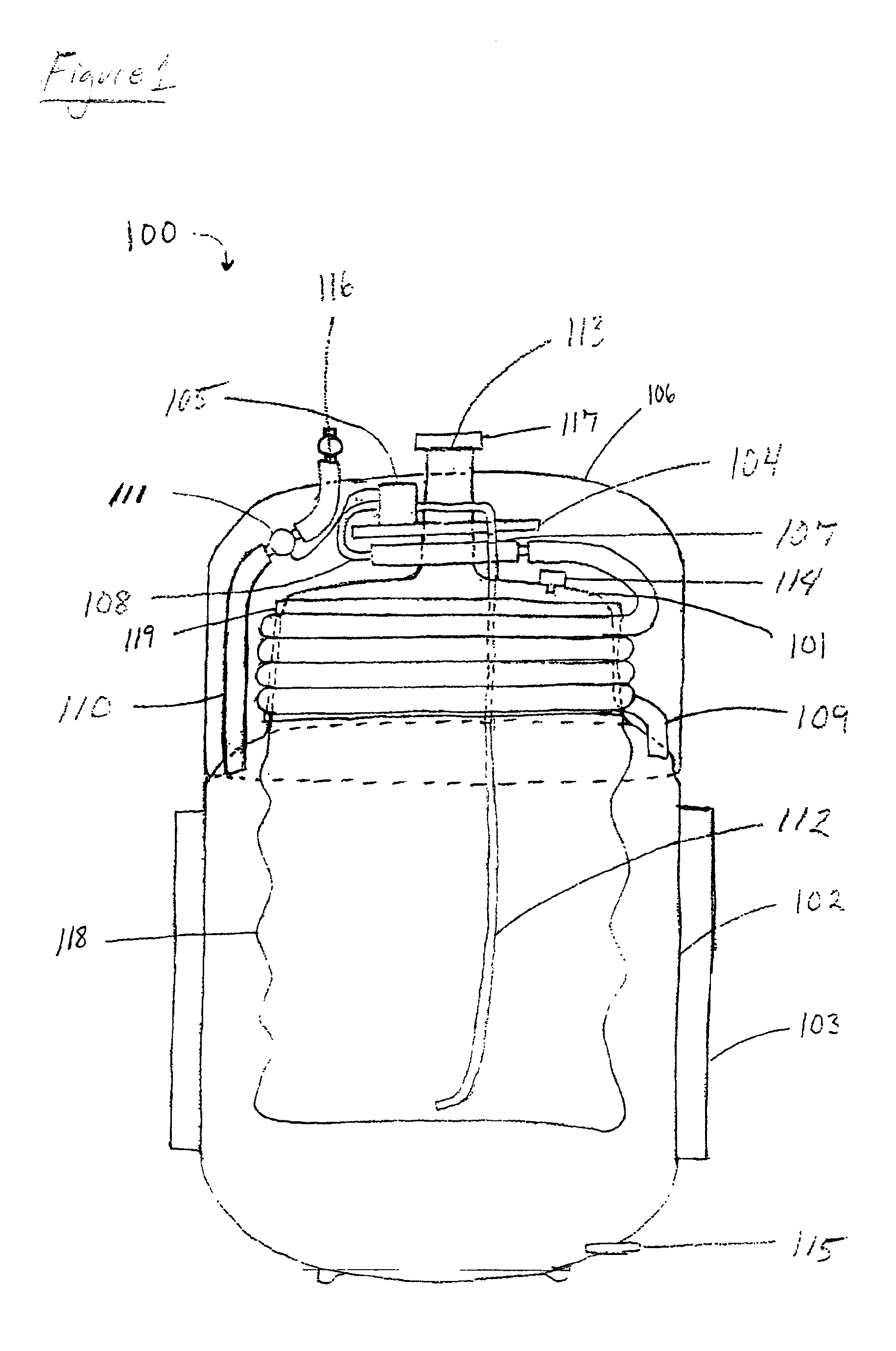
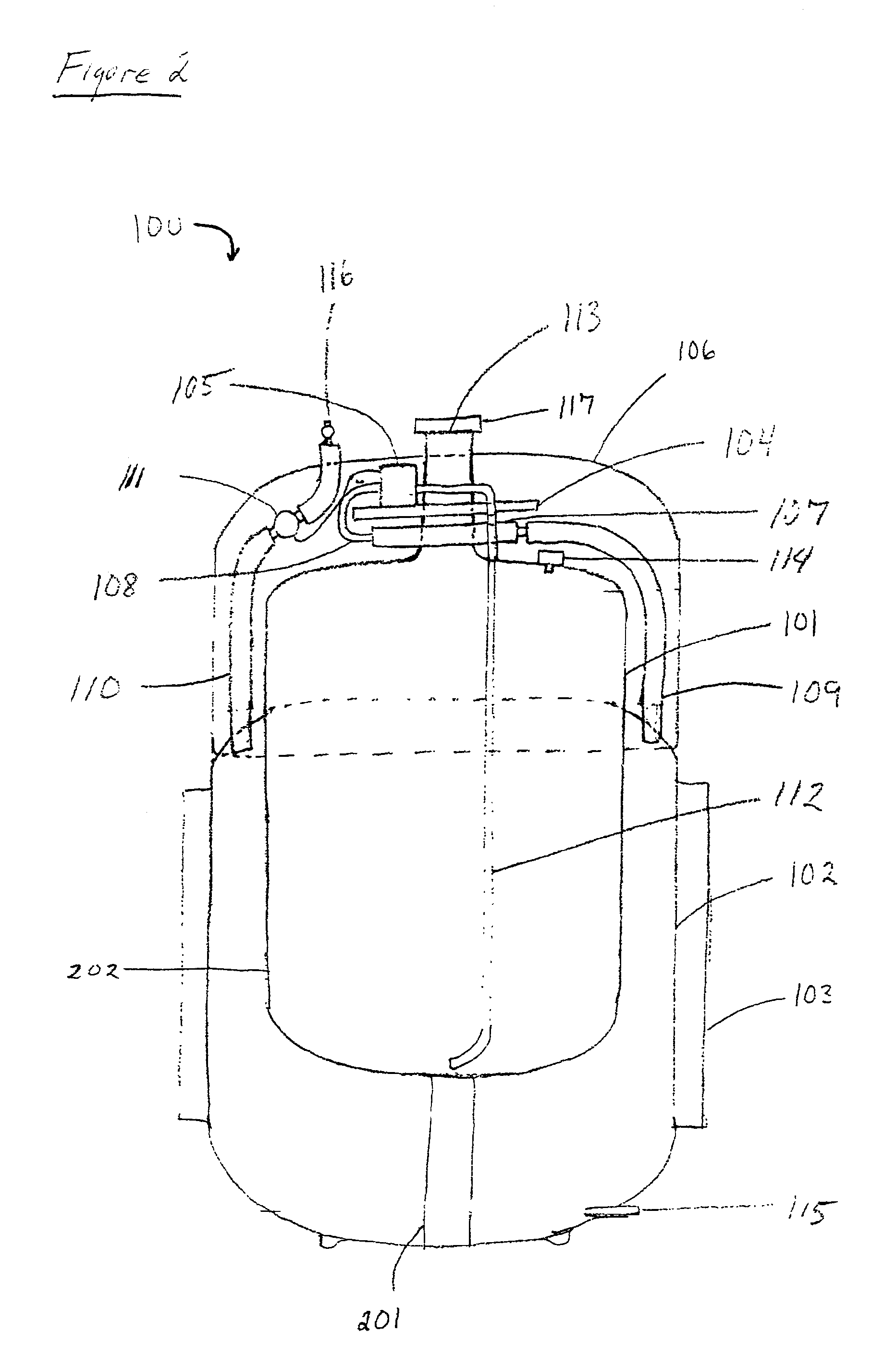
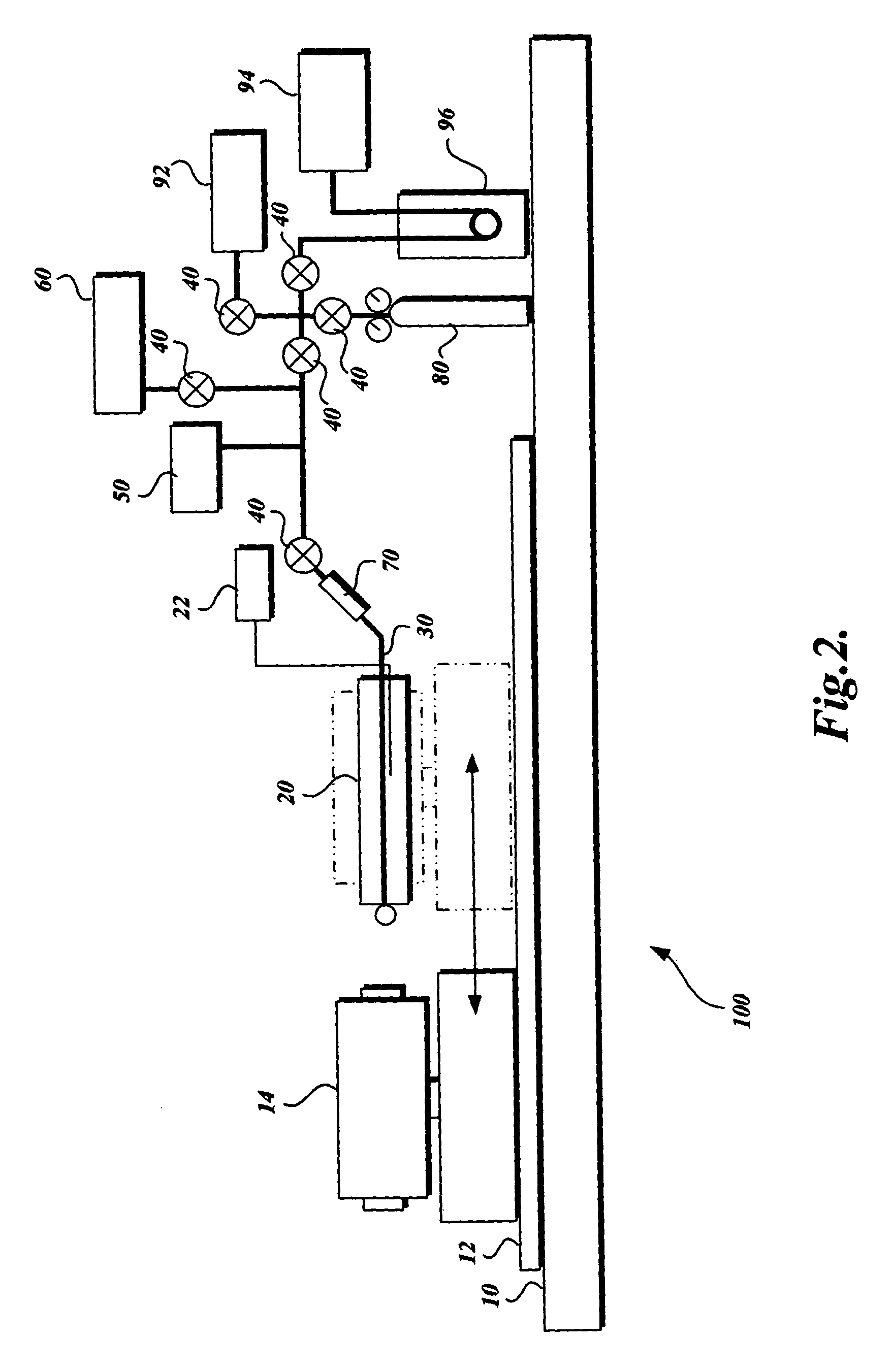
Gas storage apparatus
InactiveUS7112239B2Improve heat transfer efficiencyVessel mounting detailsReversible hydrogen uptakeCoolant flowProduct gas
A hydrogen storage apparatus that includes multiple gas storage tanks that each house a storing / adsorbing material and through the interior of which a fluid travels is provided. The gas storage apparatus 10 includes roughly cylindrical gas storage tanks 20 that house hydrogen-storing alloy. The multiple gas storage tanks 20 are disposed longitudinally parallel to each other in an ordered fashion such that roughly triangular prism-shaped empty spaces are formed between multiple adjacent hydrogen storage tanks 20. Coolant paths through which coolant flows are formed in these roughly triangular prism-shaped empty spaces. These coolant paths are thermally connected to the hydrogen-storing alloy in the gas storage tanks 20 via constituent members of the gas storage tanks 20 and via heat transfer plates 28 disposed on the gas storage tanks 20.
Owner:TOYOTA JIDOSHA KK +1
Hydrogen storage and integrated fuel cell assembly
InactiveUS20060051638A1Easy to replaceImprove heat transfer performanceFuel cell heat exchangeReactant parameters controlFuel cellsThermal insulation
Hydrogen is stored in materials that absorb and desorb hydrogen with temperature dependent rates. A housing is provided that allows for the storage of one or more types of hydrogen-storage materials in close thermal proximity to a fuel cell stack. This arrangement, which includes alternating fuel cell stack and hydrogen-storage units, allows for close thermal matching of the hydrogen storage material and the fuel cell stack. Also, the present invention allows for tailoring of the hydrogen delivery by mixing different materials in one unit. Thermal insulation alternatively allows for a highly efficient unit. Individual power modules including one fuel cell stack surrounded by a pair of hydrogen-storage units allows for distribution of power throughout a vehicle or other electric power consuming devices.
Owner:GROSS KARL J
Isoreticular metal-organic frameworks, process for forming the same, and systematic design of pore size and functionality therein, with application for gas storage
InactiveUS20050192175A1Catalyst protectionMolecular sieve catalystsSystems designMetal-organic framework
The ability to design and construct solid-state materials with pre-determined structures is a grand challenge in chemistry. An inventive strategy based on reticulating metal ions and organic carboxylate links into extended networks has been advanced to a point that has allowed the design of porous structures in which pore size and functionality can be varied systematically. MOF-5, a prototype of a new class of porous materials and one that is constructed from octahedral Zn—O—C clusters and benzene links, was used to demonstrate that its 3-D porous system can be functionalized with the organic groups, —Br, —NH2, —OC3H7, —OC5H11, —H4C2, and —H4C4, and its pore size expanded with the long molecular struts biphenyl, tetrahydropyrene, pyrene, and terphenyl. The ability to direct the formation of the octahedral clusters in the presence of a desired carboxylate link is an essential feature of this strategy, which resulted in the design of an isoreticular (having the same framework topology) series of sixteen well-defined materials whose crystals have open space representing up to 91.1% of the crystal volume, and homogeneous periodic pores that can be incrementally varied from 3.8 to 28.8 angstroms. Unlike the unpredictable nature of zeolite and other molecular sieve syntheses, the deliberate control exercised at the molecular level in the design of these crystals is expected to have tremendous implications on materials properties and future technologies. Indeed, data indicate that members of this series represent the first monocrystalline mesoporous organic / inorganic frameworks, and exhibit the highest capacity for methane storage (155 cm3 / cm3 at 36 atm) and the lowest densities (0.41 to 0.21 g / cm3) attained to date for any crystalline material at room temperature.
Owner:RGT UNIV OF MICHIGAN
Dual compartment pouch
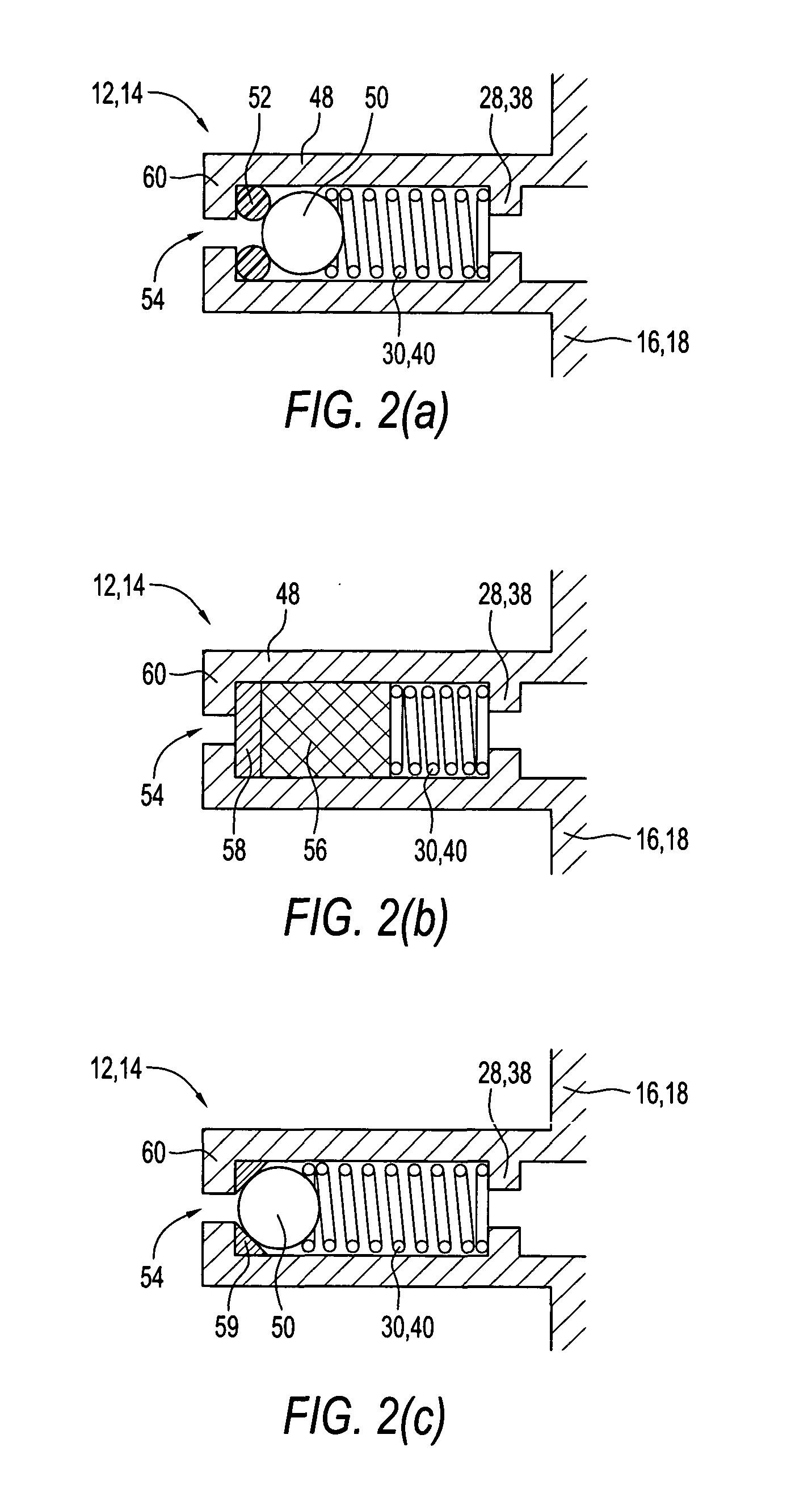
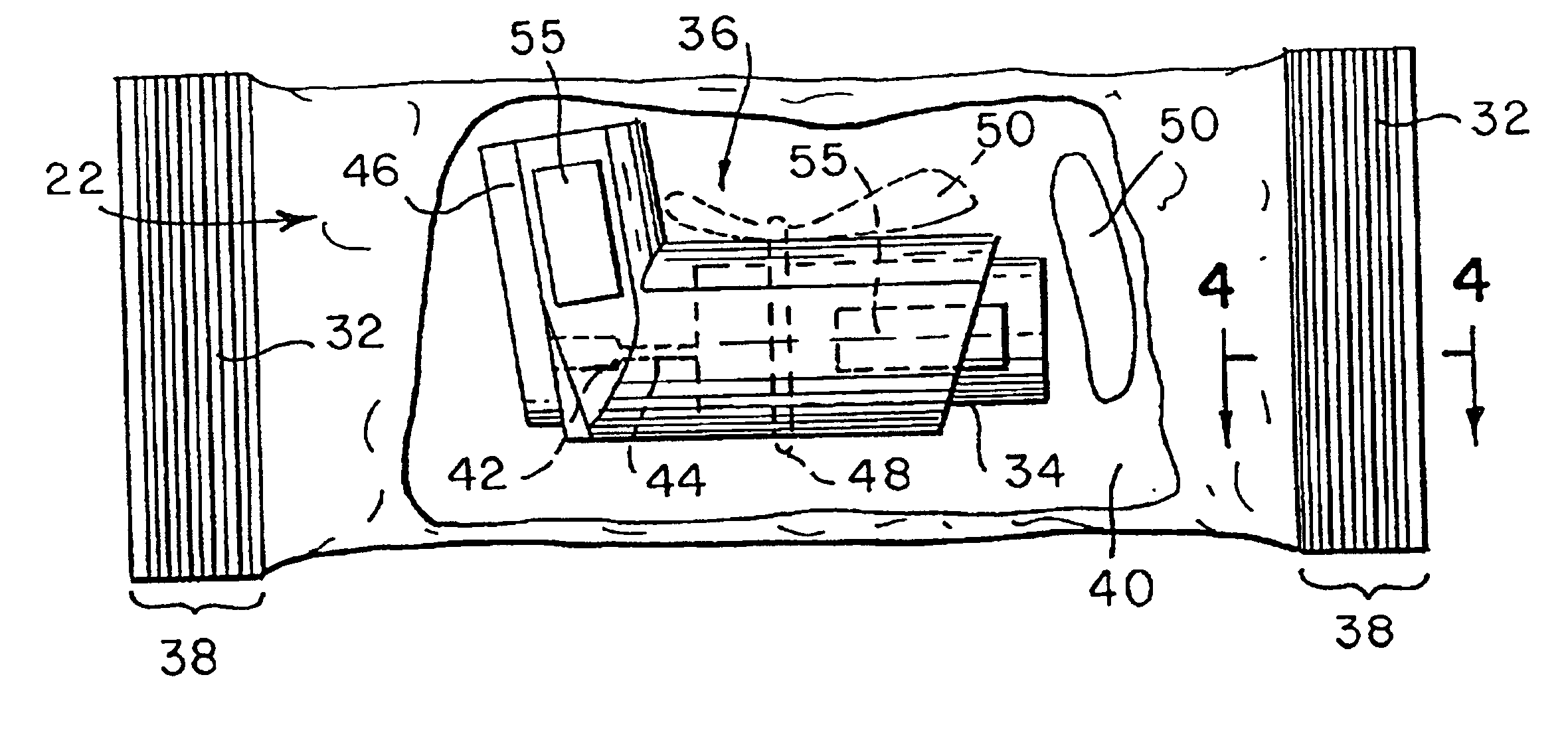
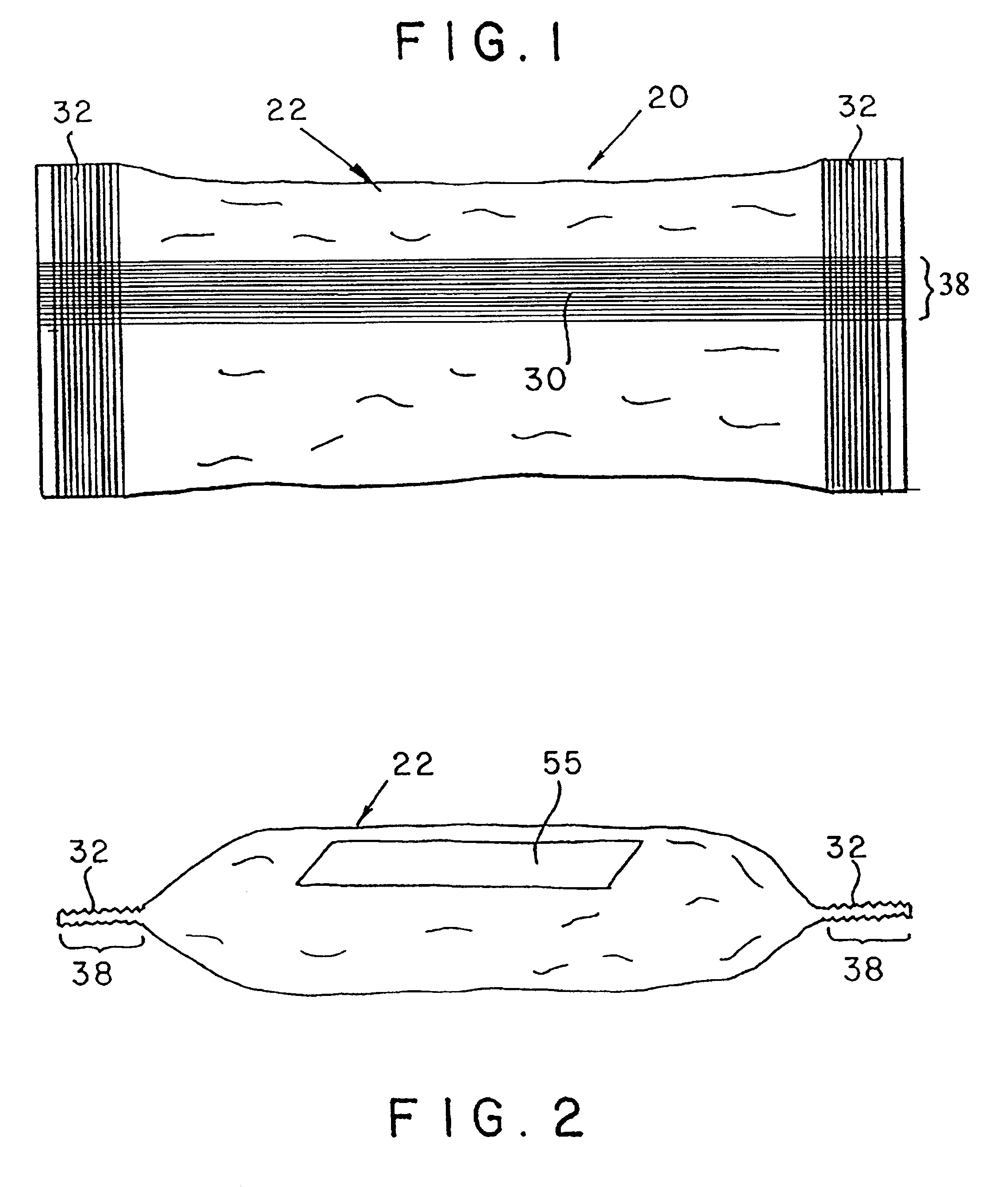
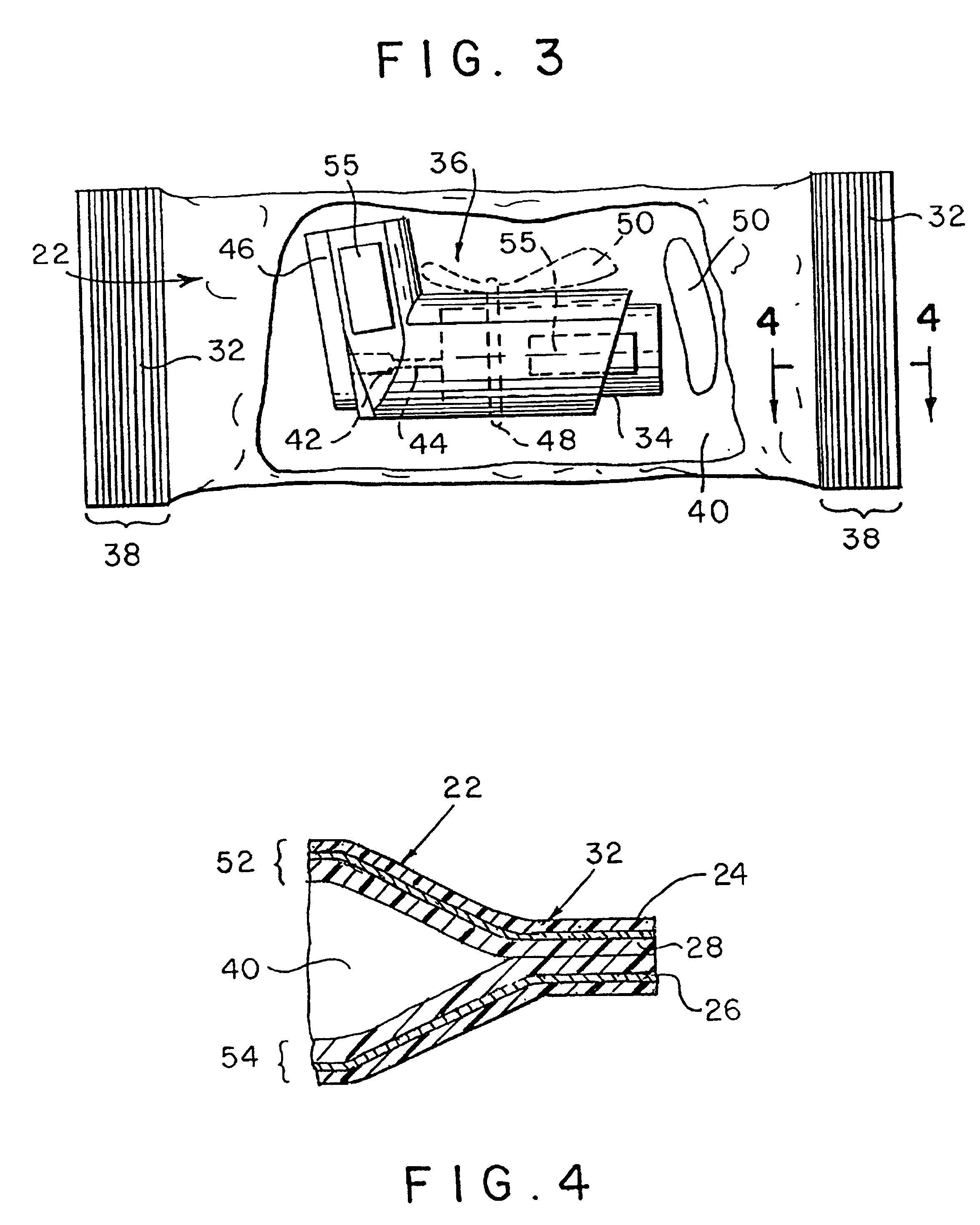
ActiveUS7631760B2Ingress of moisture vapor into the pouch can be significantly reducedDiagnosticsSurgical needlesEngineeringBiomedical engineering
The invention provides a dual compartment pouch having first and second compartments that are separated from each other by an impervious inner sheet having a breathable membrane. The breathable membrane comprises a breathable material that is pervious to moisture and gases, and impervious to liquids as well as microorganisms. The breathable membrane allows a sterilizing gas to be introduced between the first and second compartments. The front and back sheets are sealed to the inner sheet to define the first and second compartments in which the inner sheet forms a common wall between the compartments. The breathable membrane is disposed towards a central portion of the inner sheet and is spaced apart from the seams joining the front and back sheets to the inner sheet. In one embodiment, a sterilized article is disposed in one compartment, and an absorbent packets are disposed in the other compartment.
Owner:AMCOR FLEXIBLES HEALTHCARE
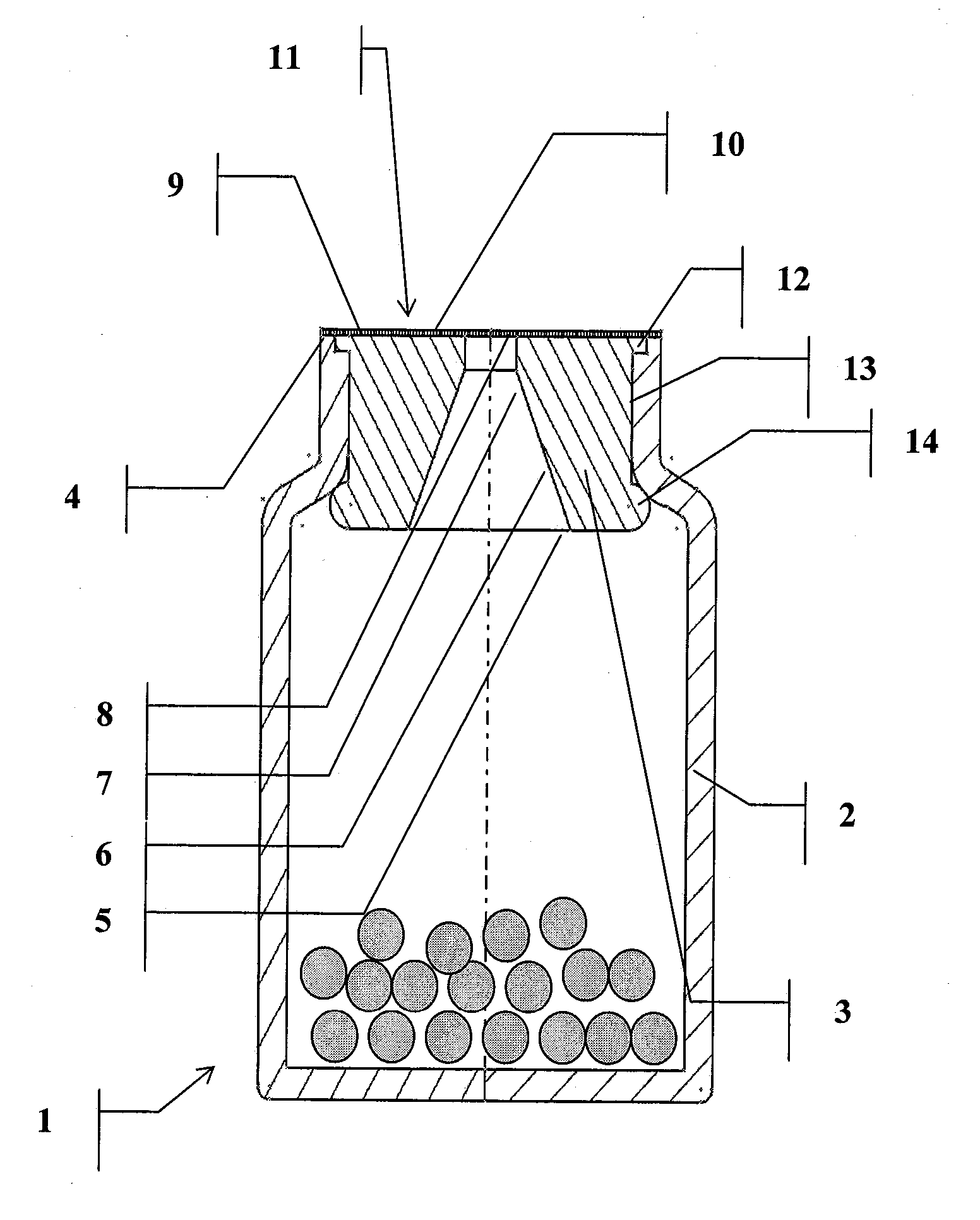
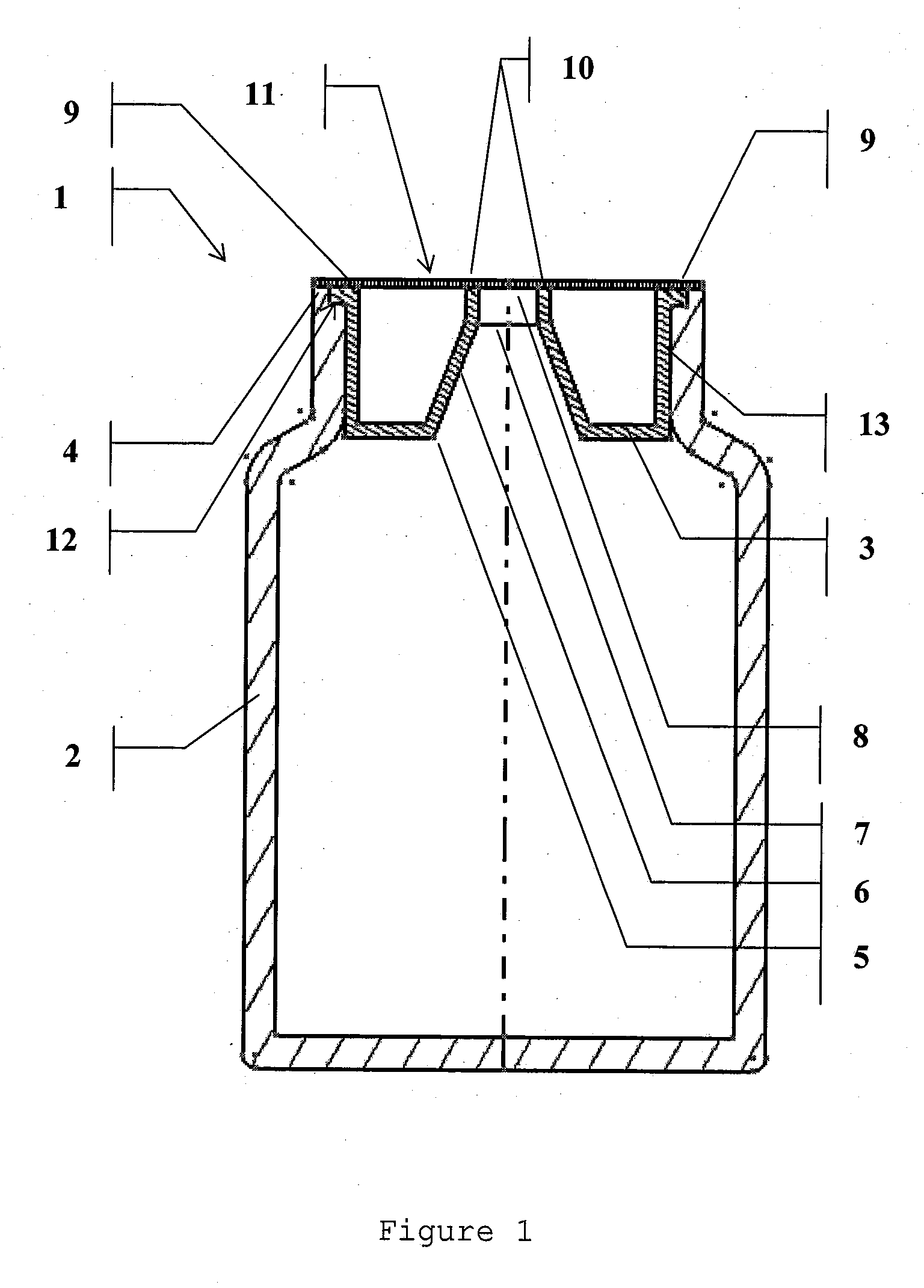
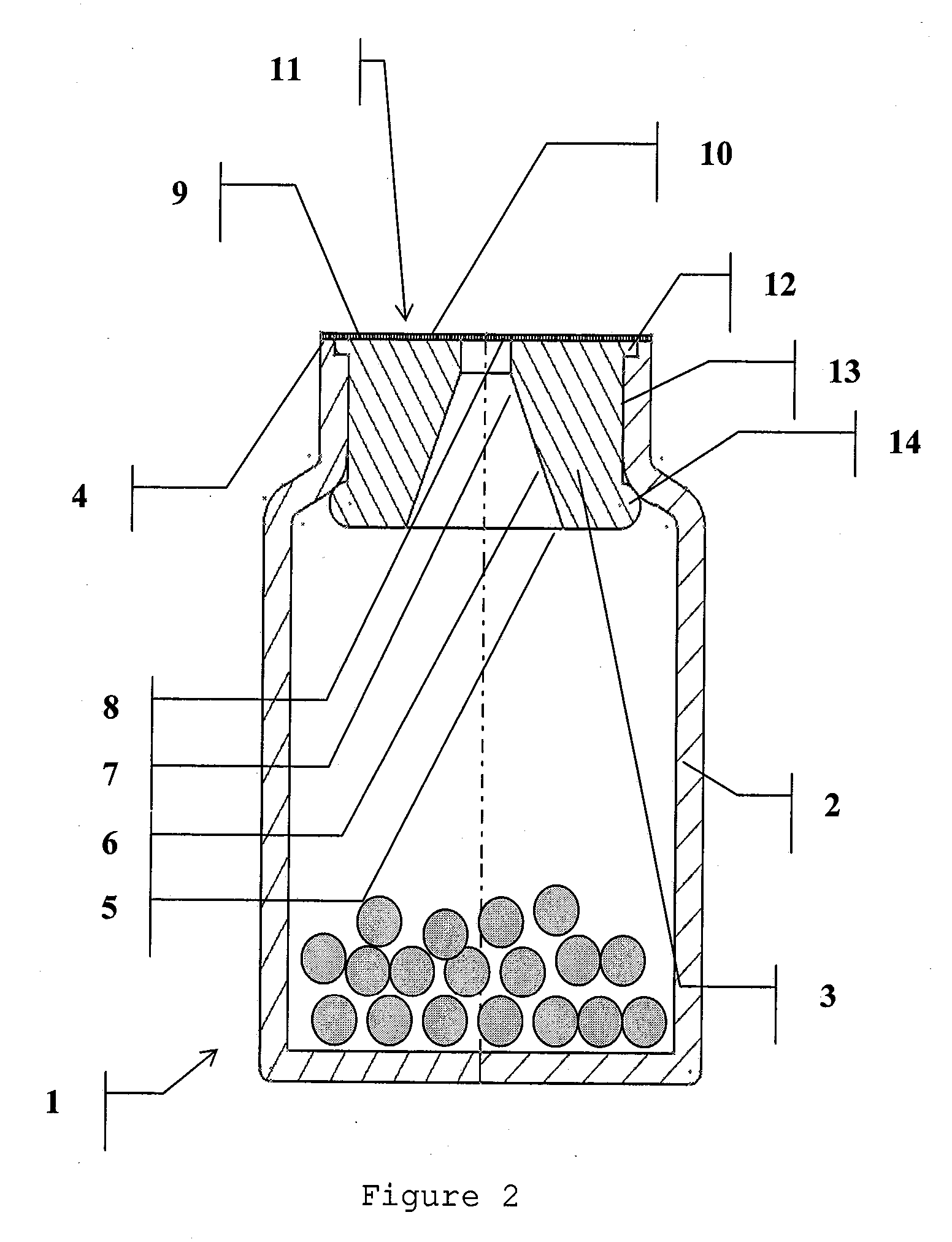
Sealed assembly for storage and distribution with discharge control for solid pharmaceutical products
The present invention relates to an assembly for the storage and distribution of solid loosely packed products unit by unit. The assembly generally comprises a storage container, a sealing means and a discharge control device inserted into the neck of the container to permit the passage of the products to be distributed. Inviolability and sealing of the container are achieved by means of a peelable heat seal, sealed thermally onto at least one surface defined by an annular, peripheral surface of the neck of the container and / or all or part of an upper surface of the device and / or a surface of a distribution opening.
Owner:AIRNOV INC
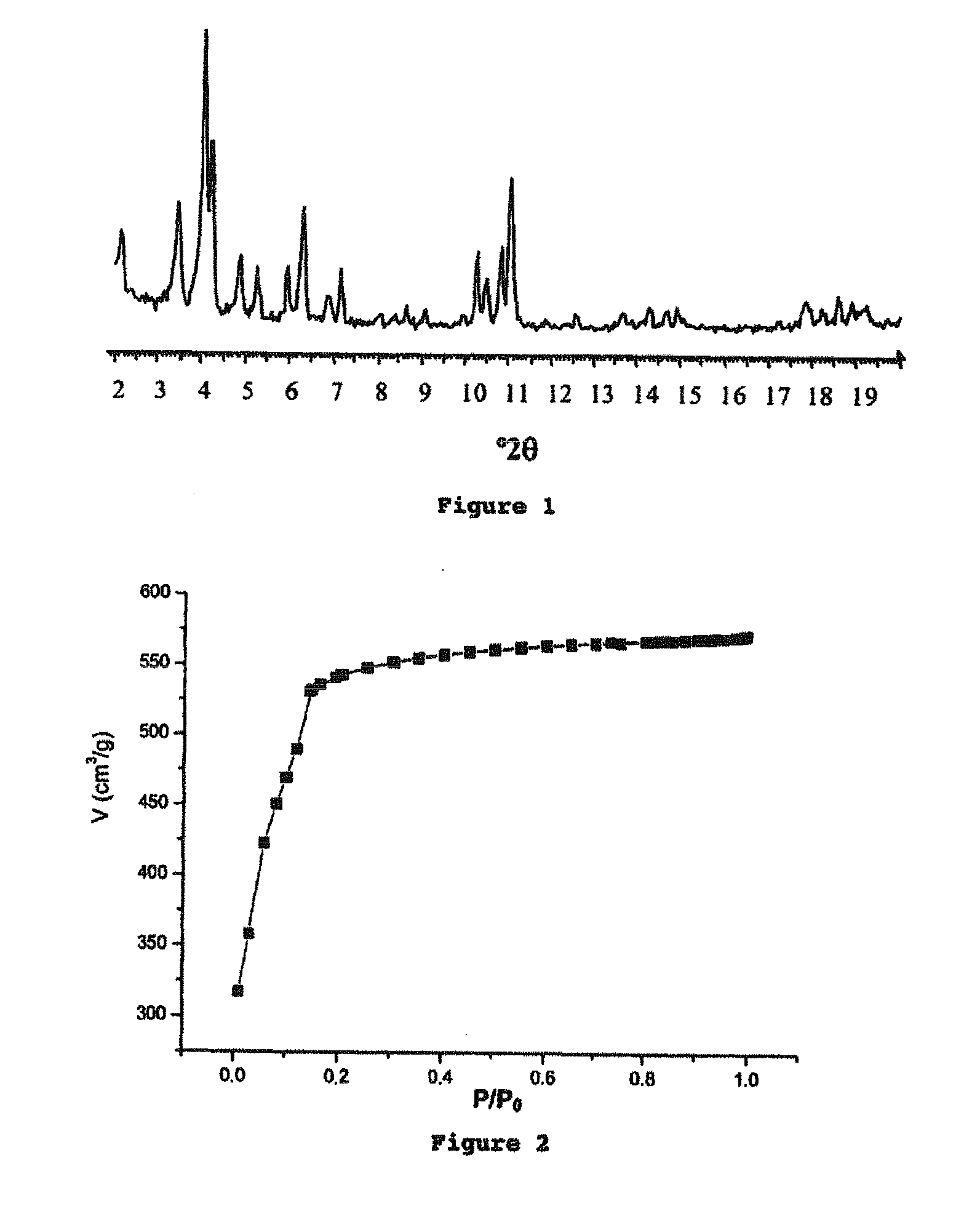
Porous crystalline hybrid solid for adsorbing and releasing gas of biological interest
The invention relates to solids made of a porous crystalline metal-organic framework (MOF) loaded with at least one gas of biological interest, and to a method for preparing the same. The MOF solids of the present invention are capable of adsorbing and releasing in a controlled manner gases having a biological interest. They can be used in the pharmaceutical field and / or for applications in the cosmetic field. They can also be used in the food industry.
Owner:CENT NAT DE LA RECHERCHE SCI +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com