Preparation method of ammonia borane-metal catalyst composite hydrogen storage material
A technology for metal catalysts and hydrogen storage materials, applied in catalyst activation/preparation, molecular sieve catalysts, chemical instruments and methods, etc., can solve problems such as troublesome operation and difficult control, achieve fast synthesis speed, inhibit release, and avoid metal ions The effect of the restoration process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1, prepare the composite hydrogen storage material of ammonia borane and metal catalyst
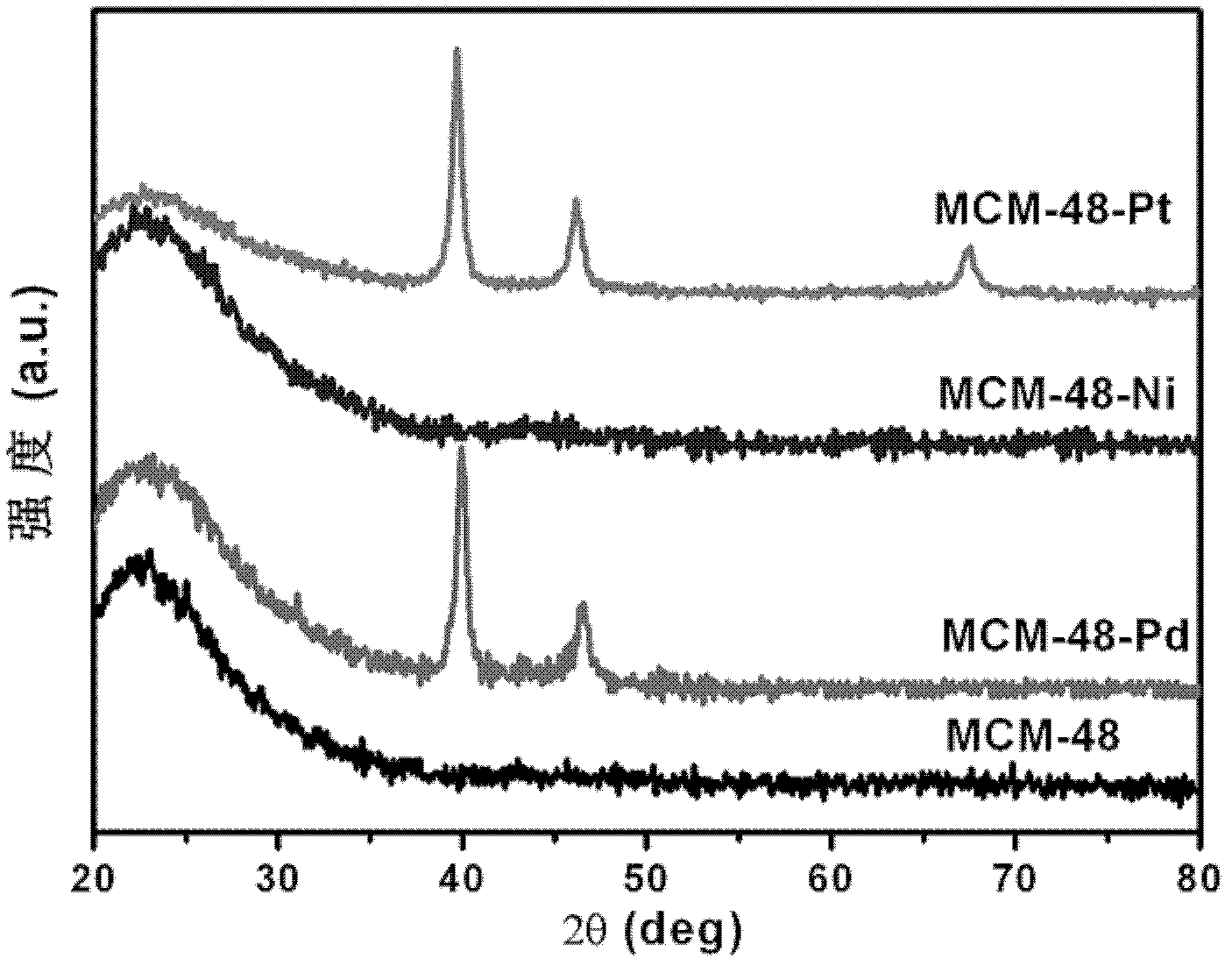
[0019] (1) The metal catalyst based on MCM-48 was synthesized by magnetron sputtering method. In this embodiment, MCM-48 is selected as the base. Before sputtering, dry the MCM-48 in a drying oven at 120°C for 2 hours to remove moisture in the material. Then put the 0.0800g MCM-48 substrate into the chamber of the magnetron sputtering apparatus and evacuate to 2×10 -4 Pa. Sputtering was performed under an argon atmosphere, the flow rate of argon gas was 76 sccm, and the working pressure was 0.7 Pa. The rotation rate of the substrate was 15 revolutions per minute. MCM-48-Pd, MCM-48-Ni and MCM-48-Pt catalysts were obtained by sputtering with Pd, Ni and Pt targets respectively for 2 minutes.
[0020] (2) Mix the catalyst obtained by sputtering with ammonia borane in a specific mass ratio (setting 1:2, 1:4 and 1:8), and then add 5 ml of water to remove the water with me...
Embodiment 2
[0023] Example 2. Research on the catalytic performance of the catalyst in the composite hydrogen storage material for the thermal decomposition of ammonia borane to decompose hydrogen
[0024] (1) Put the composite hydrogen storage material sample uniformly mixed with the catalyst prepared in Example 1 and ammonia borane in a temperature-programmed desorption-mass spectrometer.
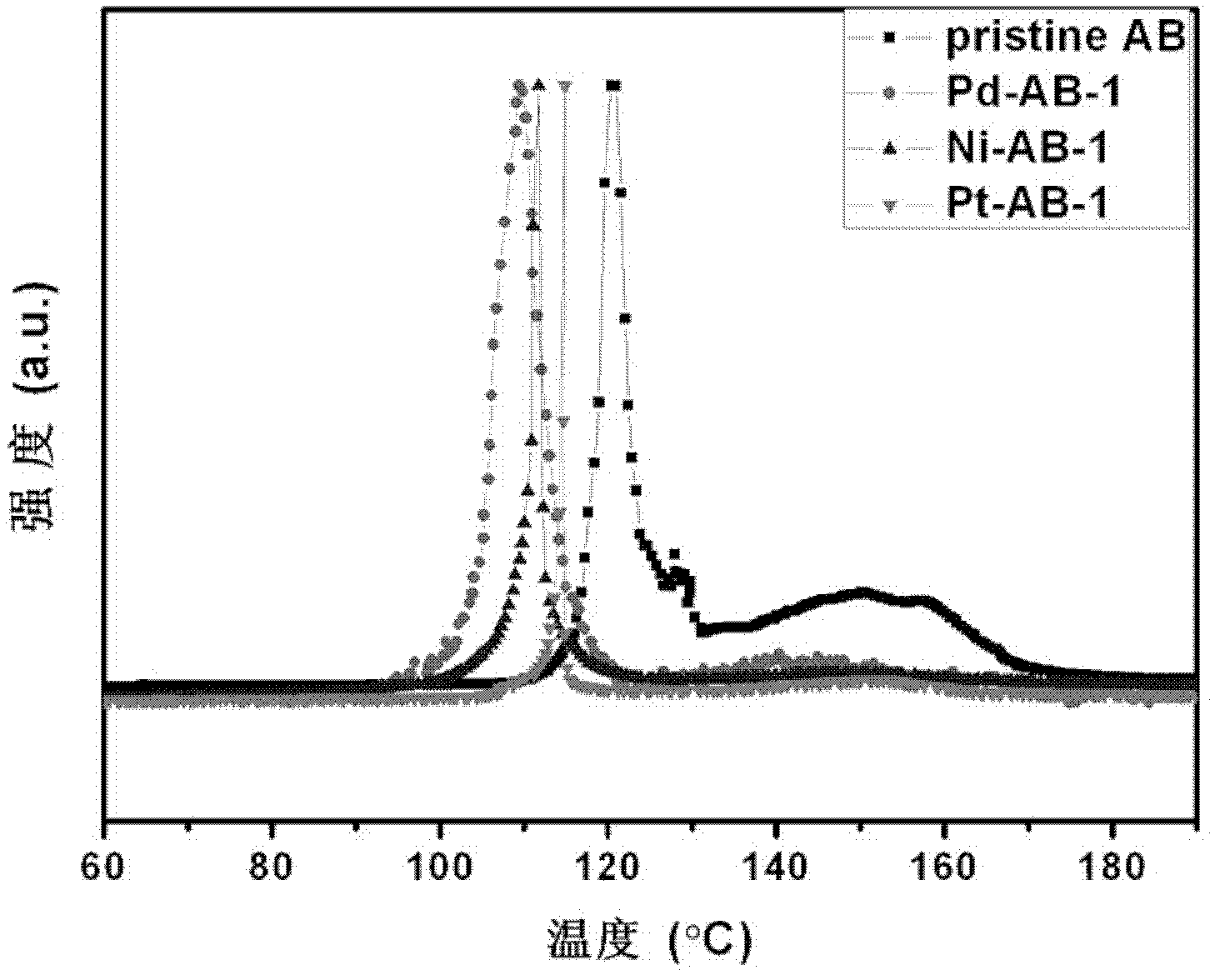
[0025] (2) Under measuring argon atmosphere, MCM-48-Pd, MCM-48-Ni and MCM-48-Pt catalyst are mixed with ammonia borane respectively by mass ratio as 1: 2 samples (respectively denoted as Pd-AB- 1. The hydrogen release temperature of Ni-AB-1 and Pt-AB-1), and the purity of the released hydrogen. Depend on image 3 It can be seen that, compared with pure ammonia borane (pristine AB), the samples after adding the catalyst all released hydrogen at a lower temperature. Among them, the catalytic performance of the sample (Pd-AB-1) composited with MCM-48-Pd and ammonia borane is particularly remarkable, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com