Multi-metal ammonia borane compound hydrogen storage material and preparation and composite hydrogen release method thereof
A metal ammonia borane and hydrogen storage material technology, which is applied in the fields of novel multi-component metal ammonia borane compound hydrogen storage materials and their preparation and composite hydrogen release, can solve the problems of reducing the theoretical hydrogen storage capacity of metal ammonia borane compounds, etc. The effect of improving energy efficiency and increasing energy density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
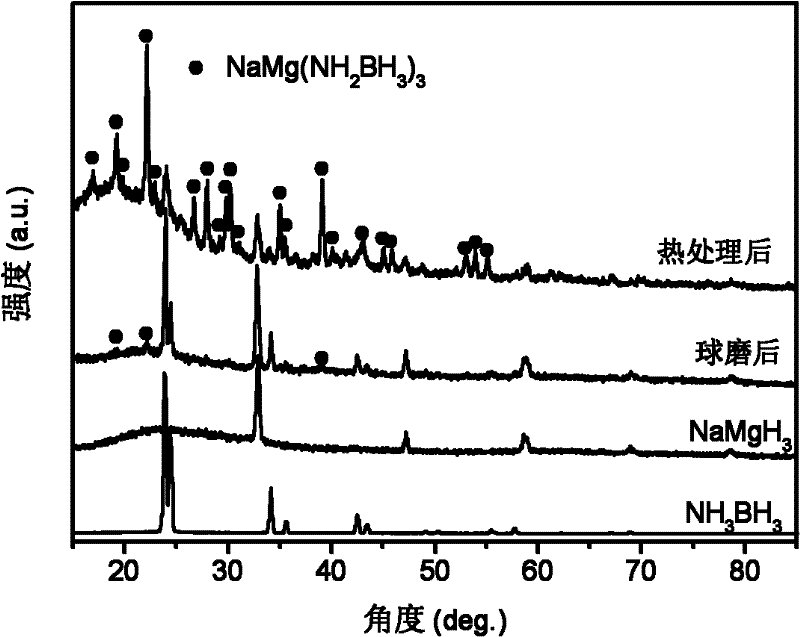
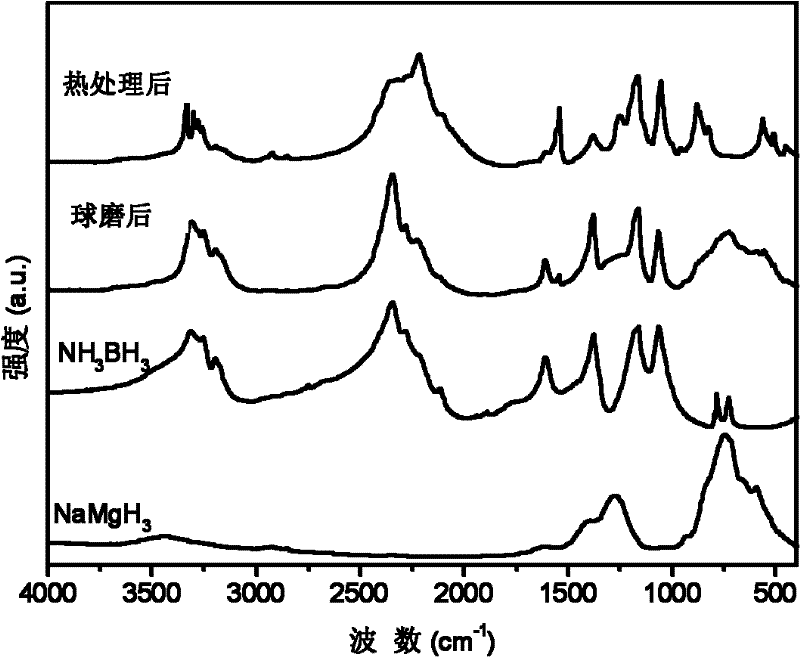
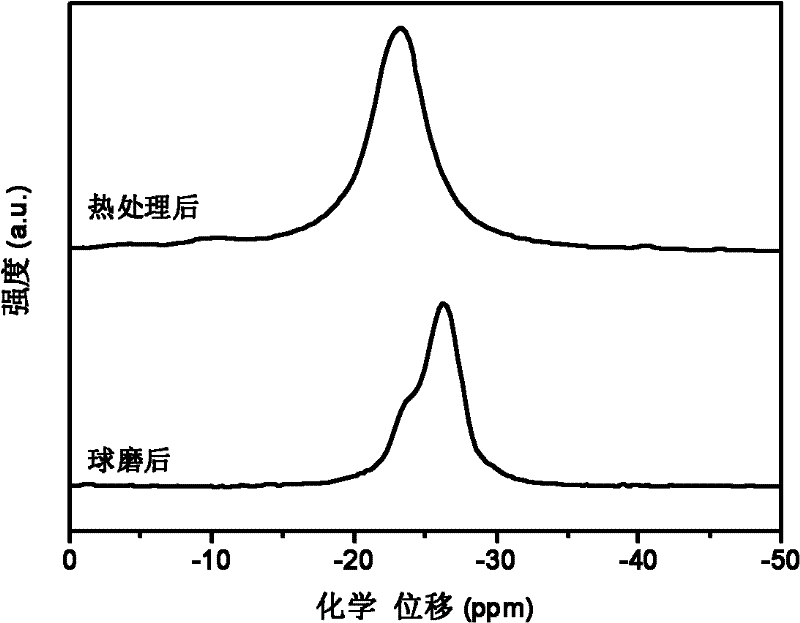
[0031] with 3NH 3 BH 3 +NaMgH 3 As the starting material, NaMg(NH 2 BH 3 ) 3 Research on the dehydrogenation performance of polymetallic ammonia borane compounds and their composites.
[0032] The raw material used is: NH 3 BH 3 (purity 97wt.%), NaMgH 3 (purity 95wt.%). In an argon atmosphere glove box, a 3:1 molar ratio of NH 3 BH 3 / NaMgH 3 The mixture and stainless steel balls were put into a stainless steel ball mill jar, sealed with a cover and then placed on a Fritsch 7 planetary ball mill for grinding for 1 hour. The ball milling atmosphere is high-purity argon (volume purity 99.9999%), the initial pressure is 1 atmosphere, and the mass ratio of balls to materials is 40:1. During the milling process, no significant pressure increase in the milling tank was found.
[0033] X-ray testing equipment and conditions: Rigaku D / max 2500, Cu Ka rays. figure 1 respectively give 3NH 3 BH 3 / NaMgH 3 (by molar ratio) The X-ray pattern of the mixture after ball milli...
Embodiment 2
[0039] with 3NH 3 BH 3 +KMgH 3 As the starting material, KMg(NH 2 BH 3 ) 3 Hydrogen storage material.
[0040] The raw material used is: NH 3 BH 3 (purity 97wt.%), KMgH 3 (purity 95wt.%). Raw material molar ratio is NH 3 BH 3 : KMgH 3 = 3:1. The ball milling time was 2 hours, and the other sample preparation conditions were the same as in Example 1. Figure 6 respectively give 3NH 3 BH 3 / KMgH 3 X-ray pattern of the sample after ball milling and heat treatment (temperature 45°C, time 20 hours). The results showed that with 3NH 3 BH 3 / NaMgH 3 The system is similar, 3NH 3 BH 3 and KMgH 3 During the ball milling process, the stability of each phase is basically maintained, and after heat treatment, KMg(NH 2 BH 3 ) 3 .
[0041] The hydrogen release performance of the material was tested by the volumetric method. Figure 7 gives 3NH 3 BH 3 / KMgH 3 Hydrogen desorption kinetics of ball milled samples at 80°C. The test results show that: due to the comp...
Embodiment 3
[0043] with 6NH 3 BH 3 +Li 3 H 6 As the starting material, Li was prepared by ball milling method 3 Al(NH 2 BH 3 ) 6 Hydrogen storage material.
[0044] The raw material used is: NH 3 BH 3 (purity 97wt.%), Li 3 H 6 (purity 95wt.%). Raw material molar ratio is NH 3 BH 3 : Li 3 H 6 =6:1. The raw materials were ball milled for 5 hours under a hydrogen atmosphere, and the other sample preparation conditions were the same as in Example 1. Figure 8 gives 6NH 3 BH 3 / Li 3 H 6 Hydrogen desorption kinetics of ball milled samples at 80°C. The test results show that: 6NH 3 BH 3 / Li 3 H 6 7 wt.% hydrogen gas was evolved within 2 minutes at 80°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com