Method for synthesizing metal coordinate hydride hydrogen-storing material directly by reaction ball milling
A hydrogen storage material and metal coordination technology, which is applied in the production of various metal hydrides, borane/diborane hydrides, hydrogen, etc., can solve the complex process, high production cost, large equipment investment, etc. problem, to achieve the effect of simple temperature requirements, low cost, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
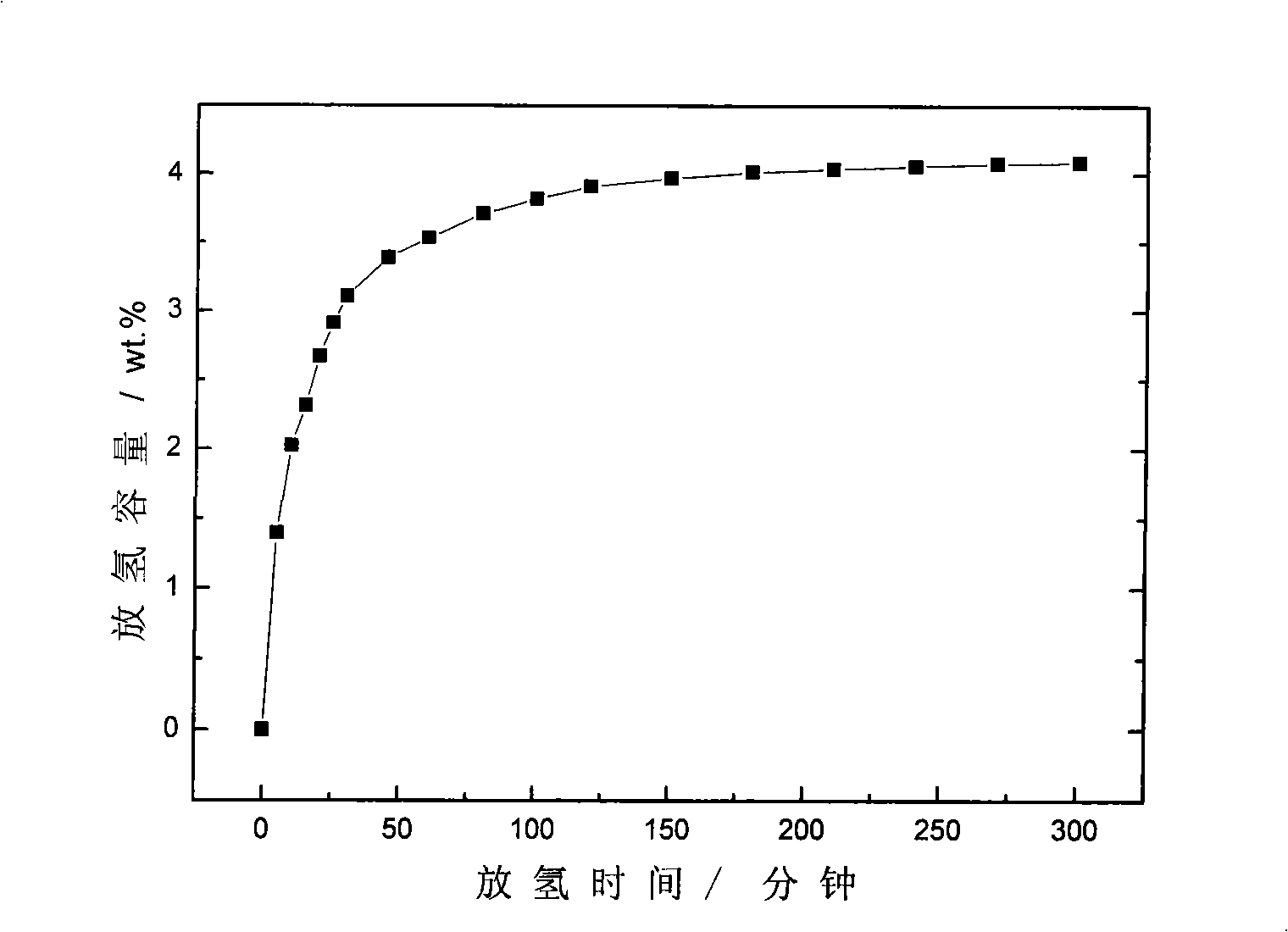
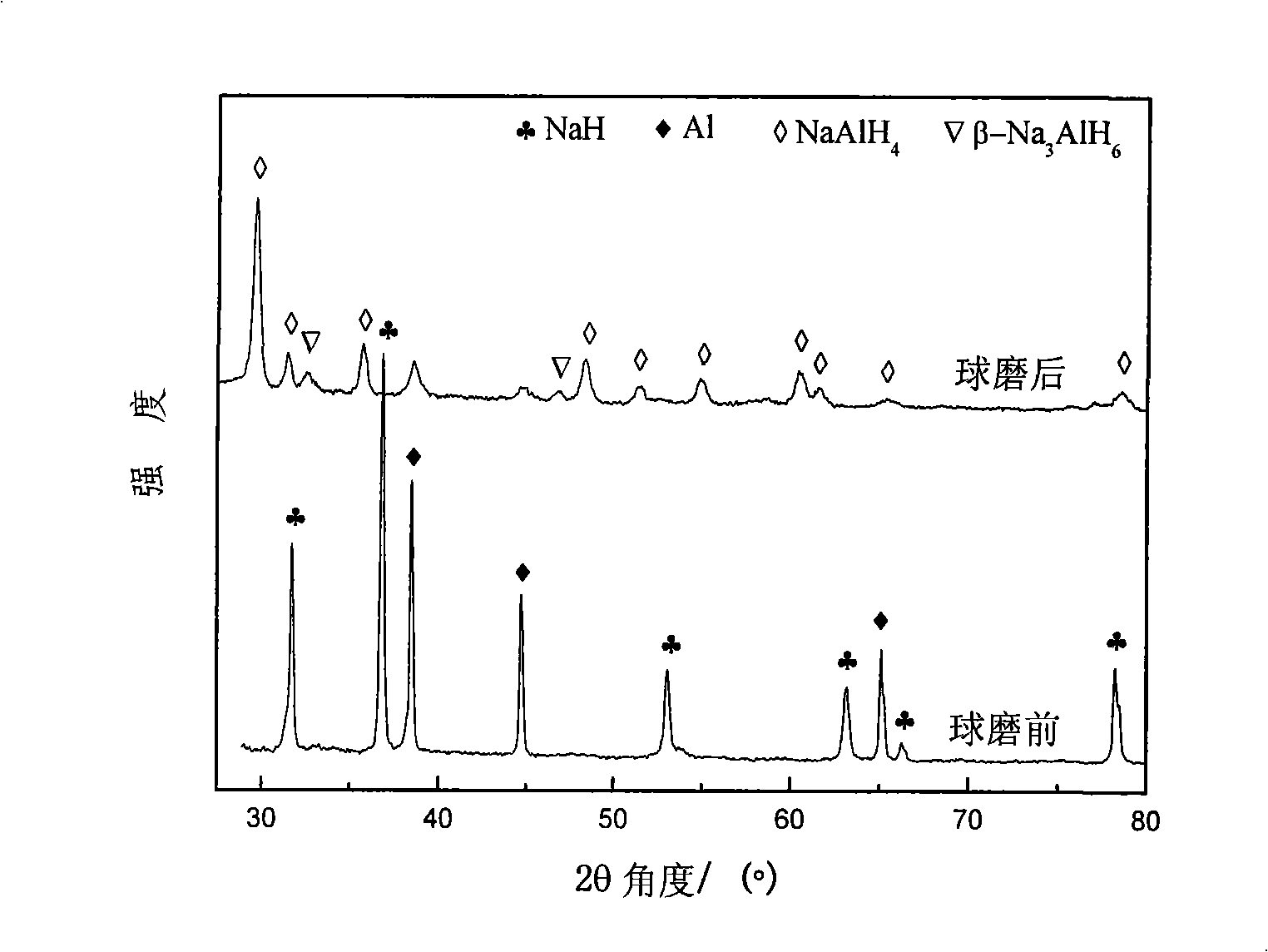
[0022] MNH according to the chemical formula 4 Coordination hydride hydrogen storage materials, choose M to be Na; N to be Al, that is, to form NaAlH 4 Coordinated hydrides. Using NaH and Al powder as raw materials, elemental Ti as catalyst, calculate the weight ratio of NaH and Al powder according to NaH:Al=1:1 (molar ratio), and then calculate Ti according to 4mol.% of the total weight of (NaH+Al) the amount of doping. The purity of the above-mentioned NaH is ≥95%, and the particle size is 74 μm; the purity of the Al powder is ≥99%, and the particle size is 74-154 μm; the purity of the Ti powder is ≥99%, and the particle size is -2 bar, then filled with hydrogen with a purity of ≥99% and 5.0 MPa, and then ball milled at room temperature for 145 hours. Prepared NaAlH 4 The hydrogen desorption capacity was measured to be 4.09wt.% for the first time.
[0023] The main chemical reactions involved in the reaction process are:
[0024]The hydrogen absorption and desorption ...
Embodiment 2
[0027] MNH according to the chemical formula 4 Coordination hydride hydrogen storage materials, choose M to be Na; N to be Al, that is, to form NaAlH 4 Coordinated hydrides. Using NaH and Al powder as raw materials, TiF 3 As a catalyst, calculate the weight ratio of NaH and Al powder according to NaH:Al=1:1 (molar ratio), and then calculate TiF according to 2mol.% of the total weight of (NaH+Al) 3 the amount of doping. The purity of the above-mentioned NaH is ≥95%, and the particle size is 74 μm; the purity of Al powder is ≥99%, and the particle size is 74-154 μm; TiF 3 Powder purity ≥ 99%, particle size ≤ 154μm. The raw material and the catalyst are put into the ball tank of the ball mill and the balls are added at a ball-to-material ratio of 30:1. Before ball milling, evacuate the spherical tank to a vacuum degree of 10 -2 bar, then filled with hydrogen with a purity of ≥99.99% and 2.5 MPa, and then ball milled at room temperature for 125 hours, the NaAlH thus prepared...
Embodiment 3
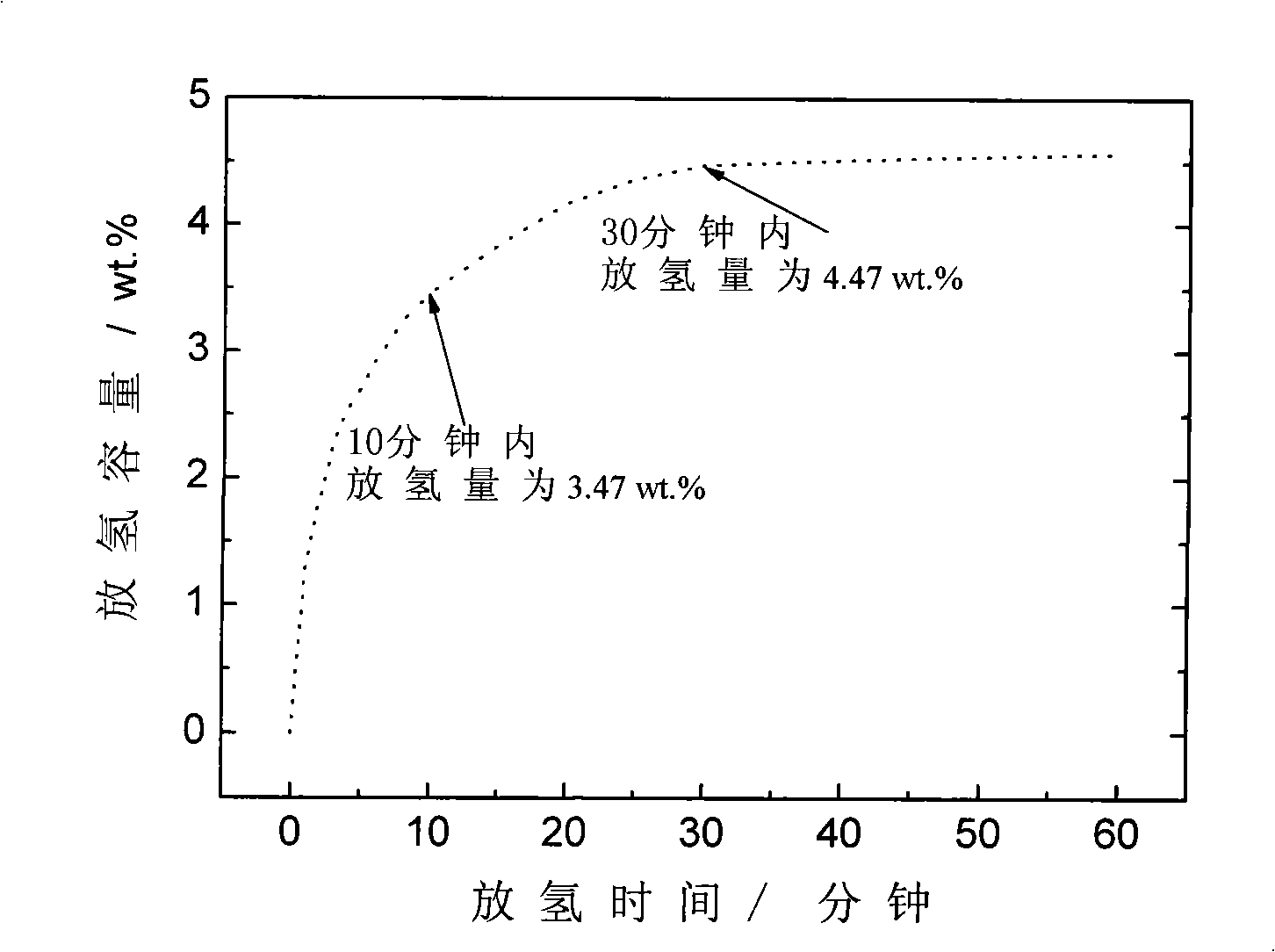
[0032] MNH according to the chemical formula 4 As a coordination hydride hydrogen storage material, M is Li; N is Al, which constitutes LiAlH 4 Coordination hydride, with LiH and Al powder as raw materials. The selected catalyst and the doping amount of the catalyst are the same as in Example 2. The purity of LiH is more than or equal to 95%, and the particle size is 74 μm; other raw material parameters are the same as in Example 1. Before ball milling, evacuate the spherical tank to a vacuum degree of 10 -2 bar, and then filled with hydrogen with a purity of ≥99.99%, 6MPa, the other reaction ball milling process was the same as in Example 2, and then ball milled at room temperature for 120h, the prepared LiAlH 4 The actual hydrogen storage capacity is 6.3wt.%.
[0033] The main chemical reactions involved in the reaction process are:
[0034] Figure 4 Doped with TiF 3 Catalyst synthesized LiAlH 4 Kinetic curves for the first hydrogen desorption of complex hydrides....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com