Device and method for producing boron trifluoride-11 electronic specific gas
A kind of technology of boron trifluoride, production method, applied in directions such as boron halide compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
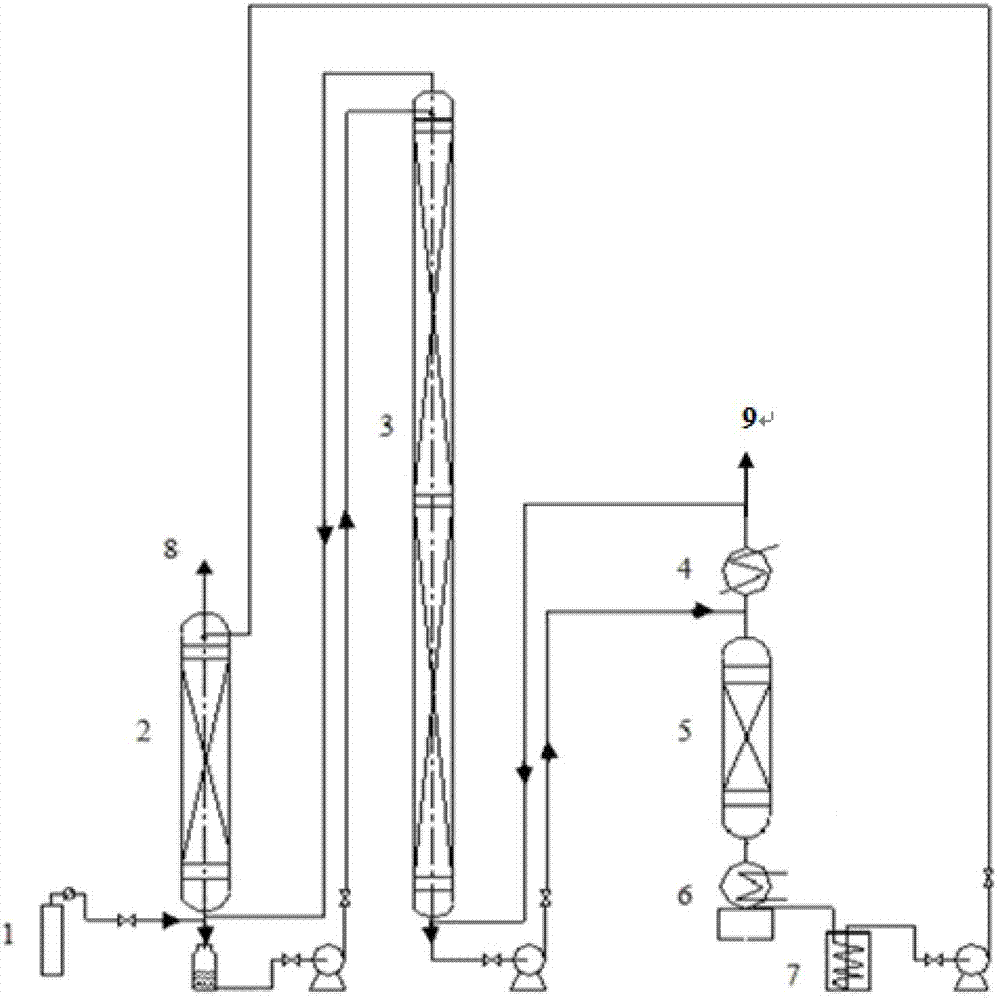
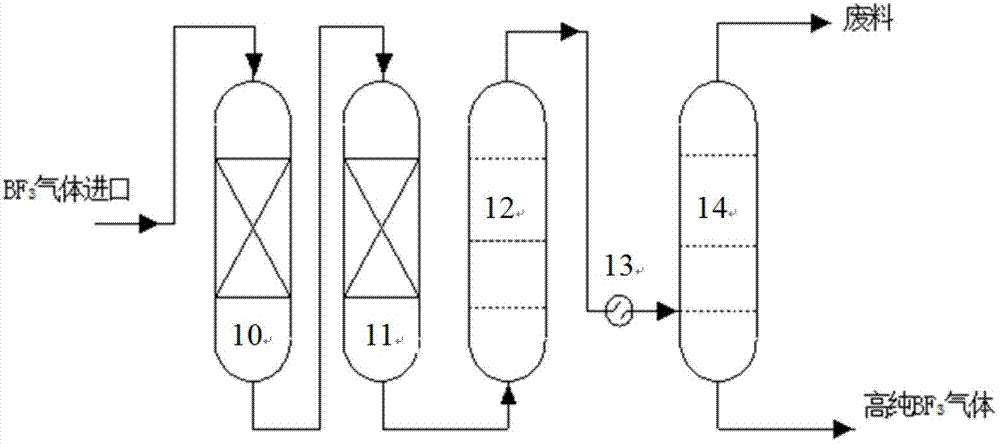
[0018] Example 1: Raw material gas cylinder 1 The boron trifluoride raw material gas enters from the bottom of the synthesis device, and anisole jets down from the top of the synthesis device. At an operating temperature of 10-15°C, a complex reaction occurs to form trifluoride Boron-anisole complex. BF cracked in cracker 3 The gas enters from the bottom of the chemical exchange tower, and the gas and liquid are fully contacted in countercurrent, and the chemical exchange reaction occurs at the operating temperature of 20-25°C. heavier 11 The B isotope is enriched at the top of the column in the form of gas, and the lighter 10 The B isotope is enriched in the liquid phase complex at the bottom of the column. enriched 11 BF 3 The gas enters the synthesis device from the bottom, and re-synthesizes a liquid-phase complex with a complexing agent. enrichment 10 The liquid-phase complex of B isotope enters the cracking device and is decomposed by heat at 140-150°C. 11 BF 3 ...
example 2
[0019]Example 2: The boron trifluoride raw material gas enters from the bottom of the synthesis device, and anisole flows down from the top of the synthesis device. At an operating temperature of 15-20°C, a complexation reaction occurs to form boron trifluoride-benzyl Ether complexes. BF cracked in cracker 3 The gas enters from the bottom of the chemical exchange tower, and the gas and liquid are fully contacted in countercurrent, and the chemical exchange reaction occurs at the operating temperature of 25-30°C. heavier 11 The B isotope is enriched at the top of the column in the form of gas, and the lighter 10 The B isotope is enriched in the liquid phase complex at the bottom of the column. enriched 11 BF 3 The gas enters the synthesis device from the bottom, and re-synthesizes a liquid-phase complex with a complexing agent. enrichment 10 The liquid-phase complex of B isotope enters the cracking device and is decomposed by heat at 150-160°C. 11 BF 3 The lean gas ent...
example 3
[0020] Example 3: Boron trifluoride raw material gas (1) enters from the bottom of the synthesis device, and anisole flows down from the top of the synthesis device. At an operating temperature of 20-25 ° C, a complexation reaction occurs to form boron trifluoride - Anisole complexes. BF cracked in cracker 3 The gas enters from the bottom of the chemical exchange tower, and the gas and liquid are fully contacted in countercurrent, and the chemical exchange reaction occurs at an operating temperature of 15-20°C. heavier 11 The B isotope is enriched at the top of the column in the form of gas, and the lighter 10 The B isotope is enriched in the liquid phase complex at the bottom of the column. enriched 11 BF 3 The gas enters the synthesis device from the bottom, and re-synthesizes the liquid-phase complex with the complexing agent. enrichment 10 The liquid-phase complex of B isotope enters the cracking device and is decomposed by heat at 160-170°C. 11 BF 3 The lean gas ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com