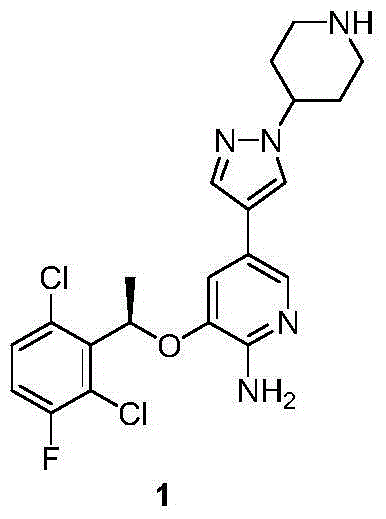
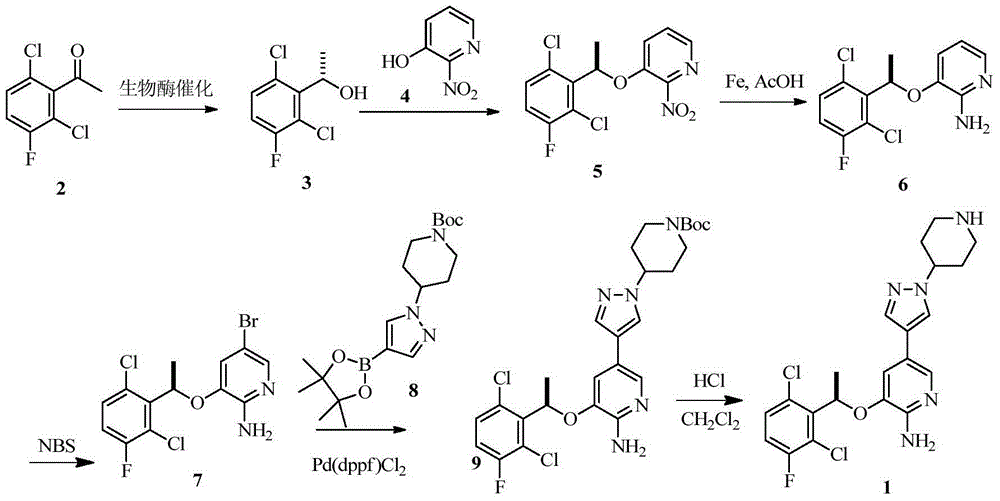
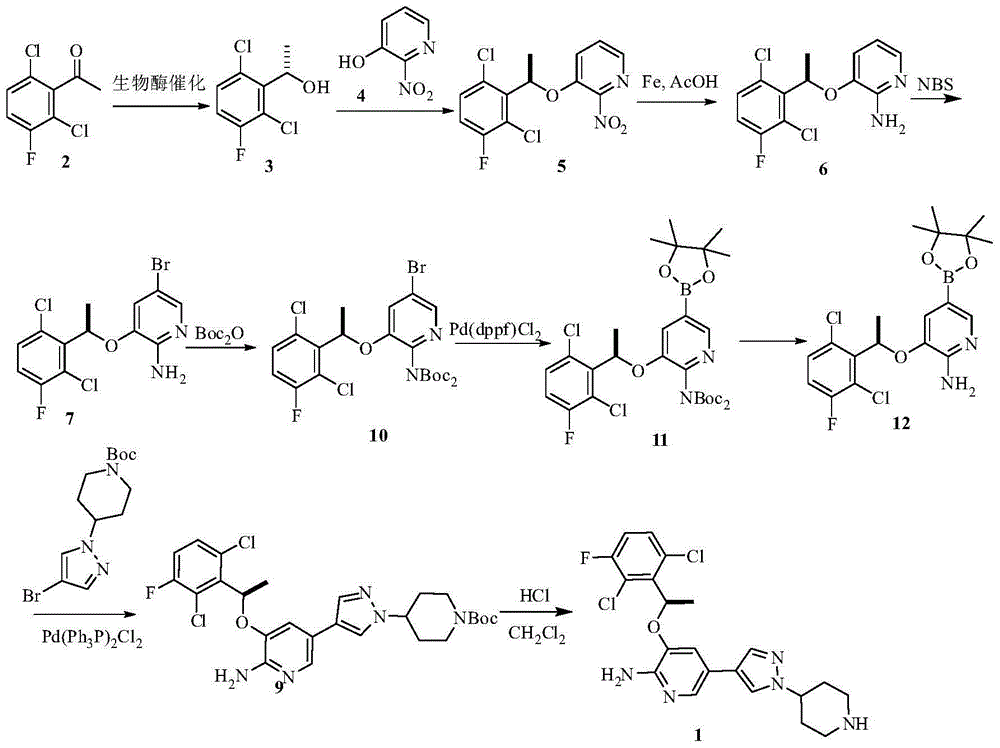
Synthesis method of crizotinib
A synthetic method and the technology of crizotinib, which are applied in the field of preparation of small molecule chemical drugs, can solve the problems of large amount of precious metal palladium catalyst, unfavorable large-scale industrial production, low reaction yield, etc., and achieve low cost, short route and high product yield. The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Compound 1 (10 g, 48 mmol) was dissolved in 1,4-dioxane (100 ml), and (-) DIPCl (22.8 g, 72 mmol) was added dropwise at -20 °C, and the reaction was kept at -20 °C for 2 h, and water (50 ml) was added. , with saturated NaHCO 3 The aqueous solution was adjusted to pH 7, extracted with ethyl acetate (30 ml x 3), dried and concentrated to give a pale yellow oil, compound 2 (8.1 g, 80% yield).
[0037] 1 H NMR (400MHz, CDCl 3 , δppm): 1.65 (d, J=6.8Hz, 3H) 5.58 (q, J=6.9Hz, 1H) 6.96-7.10 (m, 1H) 7.22-7.36 (m, 1H).
Embodiment 2
[0039]
[0040] To a solution of compound 2 (460 mg, 2.2 mmol) in THF (20 ml) were added 3-hydroxy-2-nitropyridine (350 mg, 2.42 mmol) and triphenylphosphine (880 mg, 3.2 mmol) successively under nitrogen protection. Stir at room temperature for 1 hour, then drop to 0°C, add DIAD (0.68 mL, 3.2 mmol), continue to stir and react for 12 hours, the reaction solution is spin-dried to obtain oil, which is purified by column to obtain a white solid (677 mg, 2.04 mmol, yield 93%).
[0041] 1 H NMR (400MHz, CDC l3, δppm): 1.85 (d, J=6.6Hz, 3H), 6.10 (q, J=6.6Hz, 1H), 7.04-7.13 (m, 1H), 7.21 (dd, J=8.5, 1.14Hz, 1H) , 7.30 (dd, J=9.0, 4.9Hz, 1H), 7.35-7.38 (m, 1H), 8.04 (dd, J=4.6, 1.3Hz, 1H).
Embodiment 3
[0043]
[0044] Reduced iron powder (25.5g, 0.455mol) was added to the solution of compound 3 (30g, 0.091mol) in acetic acid (200ml), and after stirring at room temperature for 5 hours, most of the acetic acid was removed, water (200ml) was added, ethyl acetate Ester extraction (3X 250ml), organic phase with saturated NaHCO 3 Wash (2×250ml), organic phase anhydrous Na 2 SO 4 After drying, it was filtered and spin-dried to obtain compound 4 (24 g, yield: 88%).
[0045] 1 H NMR (400MHz, DMSO-d6, δppm): 1.75 (d, J=6.6Hz, 3H), 5.67 (brs, 2H), 5.97-5.92 (q, J=6.6Hz, 1H), 6.38-6.35 (dd , J=5.0Hz, 7.7Hz, 1H), 6.61 (d, J=7.1Hz, 1H), 7.47-7.42 (m, 2H), 7.56-7.52 (dd, J=5.0Hz, 7.7Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com