Industrial production method of boron-10 isotope
An isotope and purpose technology, applied in the field of chemical synthesis and separation, can solve problems such as corrosion of equipment, blockage of pipelines, heat exchangers, production cannot be carried out continuously and stably, and achieve the effect of continuous and stable production and reduction of side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
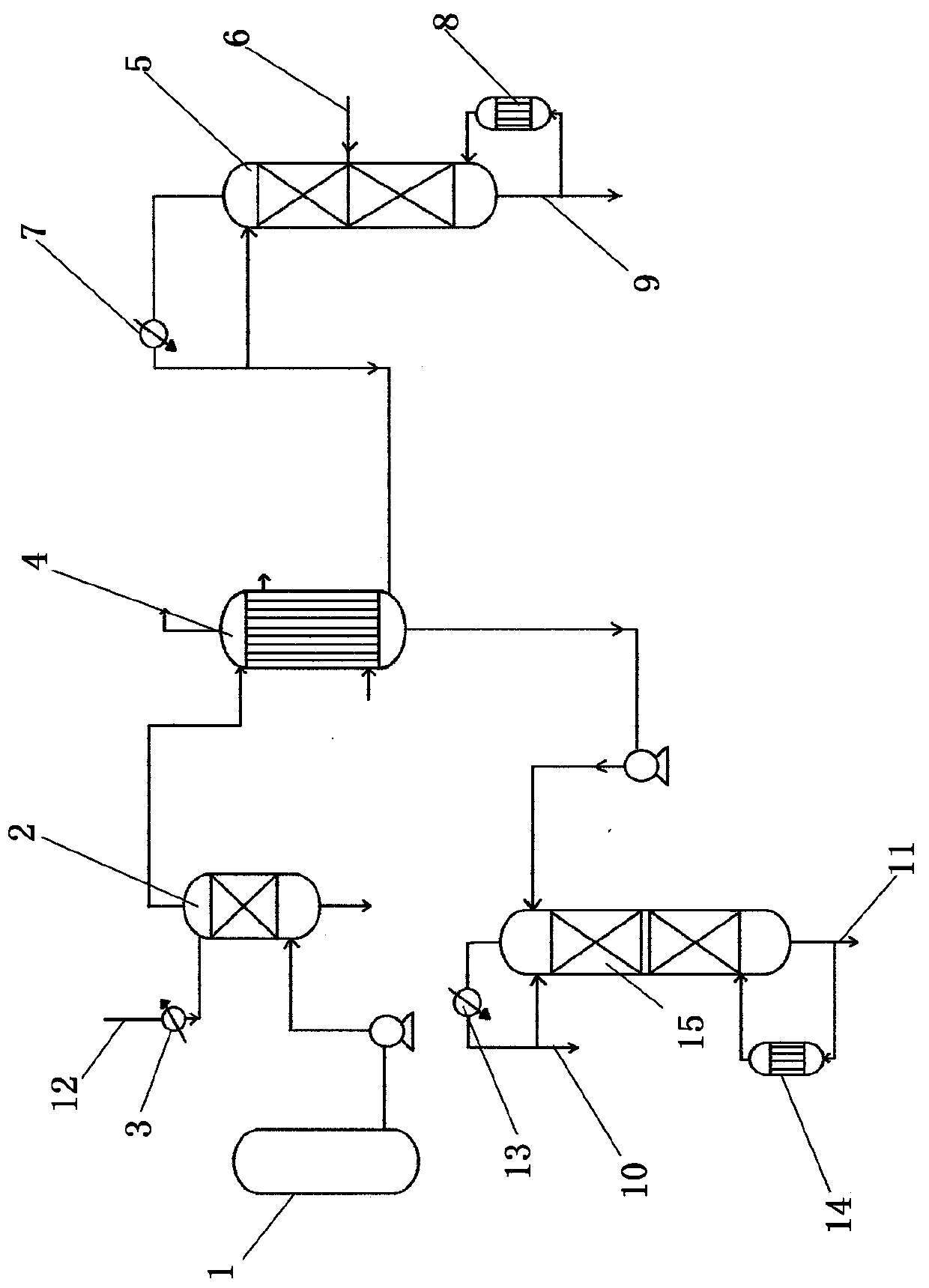
[0031] The raw material methyl ether liquid is pumped to the molecular sieve adsorption tower, and passes through the molecular sieve bed from the lower part of the molecular sieve adsorption tower upwards, and the residence time is 30 minutes. The adsorption process was carried out at 0.5MpaG and 0°C. After molecular sieve adsorption treatment, the water content in the refined methyl ether was reduced to 0.001%. The raw boron trifluoride gas enters the low-temperature rectification tower, the temperature at the top of the tower is controlled at -105°C, and the pressure is controlled at O.1MpaG. After rectification, refined boron trifluoride comes out from the top of the tower, free of hydrogen fluoride and tetrafluoride Impurities such as silicon. Refined methyl ether flows through the complexation reactor tubes from top to bottom, refined boron trifluoride gas enters the complexation reactor tubes from bottom to top, methyl ether and boron trifluoride undergo complexation r...
Embodiment 2
[0033] The raw material methyl ether liquid is pumped to the molecular sieve adsorption tower, and passes through the molecular sieve bed from the lower part of the adsorption tower upwards, and the residence time is 45 minutes. The adsorption process was carried out at 0.1MpaG and -30°C. After molecular sieve adsorption treatment, the water content in the refined methyl ether was reduced to 0.0018%. The raw boron trifluoride gas enters the low-temperature rectification tower, the temperature at the top of the tower is controlled at -60°C, and the pressure is controlled at 0.8MpaG. After rectification, refined boron trifluoride comes out from the top of the tower, free of hydrogen fluoride and tetrafluoride Impurities such as silicon. Refined methyl ether flows through the complexation reactor tubes from top to bottom, refined boron trifluoride gas enters the complexation reactor tubes from bottom to top, methyl ether and boron trifluoride undergo complexation reaction on the...
Embodiment 3
[0035] The raw material methyl ether liquid is pumped to the molecular sieve adsorption tower, and passes through the molecular sieve bed layer upwards from the lower part of the adsorption tower, and the residence time is 60 minutes. The adsorption process was carried out at 1.0MpaG and 20°C. After molecular sieve adsorption treatment, the water content in the refined methyl ether was reduced to 0.002%. The raw boron trifluoride gas enters the low-temperature rectification tower, the temperature at the top of the tower is controlled at -85°C, and the pressure is controlled at 1.2MpaG. After rectification, refined boron trifluoride comes out from the top of the tower, free of hydrogen fluoride and silicon tetrafluoride and other impurities. Refined methyl ether flows through the complexation reactor tubes from top to bottom, refined boron trifluoride gas enters the complexation reactor tubes from bottom to top, methyl ether and boron trifluoride undergo complexation reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com