Preparation method of high-purity enriched 11B boron trifluoride gas
A technology of boron trifluoride and boron trifluoride methyl ether, which is applied in the direction of boron halide compounds, can solve the problems of long and cumbersome process routes, and achieve the effects of long process routes, simple process and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
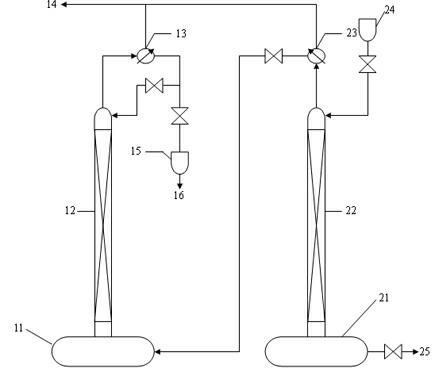
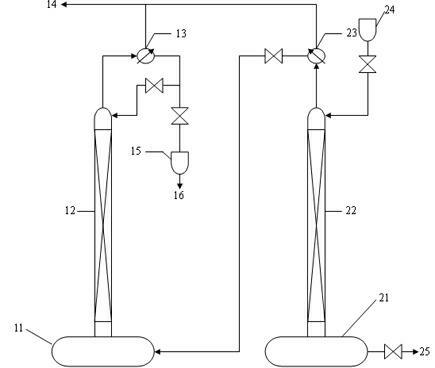
[0025] preparation 11 B abundance 95% (at.) high purity 11 B boron trifluoride gas, raw materials used for production enrichment 10 produced in the course of B 11 B boron trifluoride methyl ether complex ( 11 B abundance ≥ 90% (at.)), using the chemical exchange method to enrich in the high-efficiency distillation tower in series with two towers 11 b. Make 11 The abundance of B reaches 95% (at.), thus 11 Prepared by direct reaction of B complex with sodium fluoride 11 B sodium fluoroborate, then by 11 B sodium fluoroborate is thermally decomposed 11 B boron trifluoride gas. Prepared 11 B boron trifluoride gas, measured 11 The abundance of B is 95.5% (at.), and the purity is 99.99vol%;
[0026] Preparation of the present invention 11B abundance 95% (at.) high purity 11 B boron trifluoride gas, the preparation method steps are as follows:
[0027] (1) Enrichment 11 B abundance reaches 95% (at.): Separation and enrichment 11 The B isotope is carried out in a ...
Embodiment 2
[0031] preparation 11 B abundance 97% (at.) high purity 11 B boron trifluoride gas, raw materials used for production enrichment 10 produced in the course of B 11 B boron trifluoride methyl ether complex ( 11 B abundance ≥ 90% (at.)), using the chemical exchange method to enrich in the high-efficiency distillation tower in series with two towers 11 b. Make 11 The abundance of B reaches 97% (at.), thus 11 Formed by reaction of B complex with sodium fluoride 11 B sodium fluoroborate, reheated 11 B sodium fluoroborate is decomposed 11 B boron trifluoride gas. Prepared 11 B boron trifluoride gas 11 The abundance of B is 97.3% (at.), and the purity is 99.99vol%;
[0032] The preparation method steps are as follows:
[0033] (1) Enrichment 11 B abundance reaches 97% (at.): Separation and enrichment 11 The B isotope is carried out in a high-efficiency distillation tower with two towers connected in series. The height of the tower is 13 meters, and the inner diame...
Embodiment 3
[0037] preparation 11 B abundance 99% (at.) high purity 11 B boron trifluoride gas, raw materials used for production enrichment 10 produced in the course of B 11 B boron trifluoride methyl ether complex ( 11 B abundance ≥ 90% (at.)), using the chemical exchange method to enrich in the high-efficiency distillation tower in series with two towers 11 b. Make 11 The abundance of B reaches 99% (at.), thus 11 Formed by reaction of B complex with sodium fluoride 11 B sodium fluoroborate, reheated 11 B sodium fluoroborate is decomposed 11 B boron trifluoride gas. Prepared 11 B boron trifluoride gas 11 The abundance of B is 99.2% (at.), and the purity is 99.99vol%;
[0038] The preparation method steps are as follows:
[0039] (1) Enrichment 11 The abundance of B reaches 99% (at.), separation and enrichment 11 The B isotope is carried out in a high-efficiency distillation tower with two towers connected in series. The height of the tower is 16 meters, and the inn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com