One alkyl triphenyl substituted group based phosphonium salt preparation method and application
A technology of alkyl triphenyl and quaternary phosphonium salts, which is applied to the preparation of organic compounds, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc., and can solve the problem of poor thermal stability, which is only 40%, High thermal stability, long active time, and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
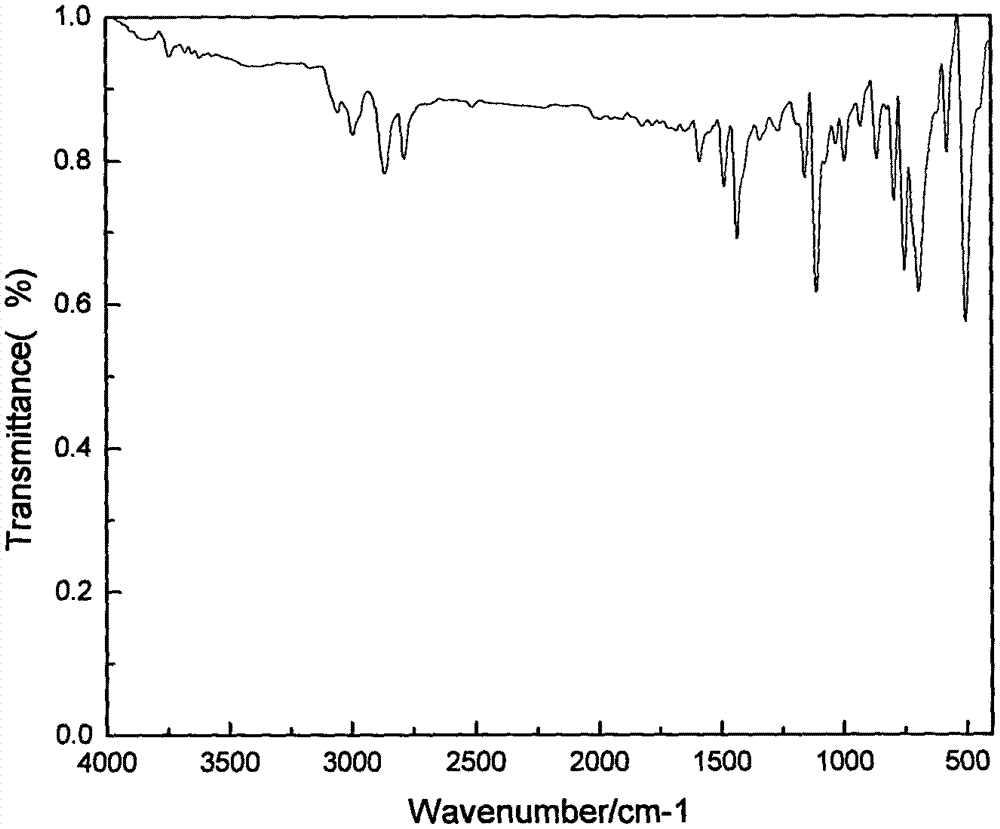
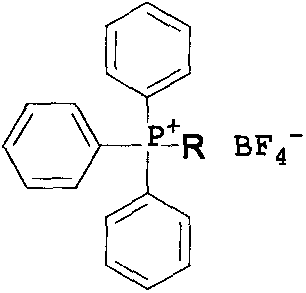
[0034] A quaternary phosphonium salt containing a monoalkyl triphenyl substituent and a preparation method thereof. The quaternary phosphonium salt containing an alkyl triphenyl substituent is denoted as II 1 , whose structural formula is:
[0035]
[0036] The quaternary phosphonium salt containing multiple alkyl triphenyl substituents is denoted as II 1 The concrete steps of the preparation method are:
[0037] Step 1, quaternary phosphonium halide I 1 preparation of
[0038] Quaternary Phosphonium Halide I 1 The structural formula is:
[0039]
[0040] Quaternary Phosphonium Halide I 1 Preparation: Add 7.86g (0.03mol) of triphenylphosphine and 20ml of acetonitrile into a 100ml three-neck flask equipped with a condenser tube, add 6mL (0.06mol) of bromoethane dropwise at constant pressure under nitrogen protection, reflux and stir for 10 hours, and react After completion, a uniform yellow oily liquid was obtained, which was cooled in the refrigerator. After the w...
Embodiment 2
[0049] A kind of quaternary phosphonium salt II containing an alkyl triphenyl substituent 1 the use of. The quaternary phosphonium salt II 1 As a phase transfer catalyst for fluorine-chlorine exchange reaction, the specific steps are:
[0050] Step 1. Take a certain amount of SD-KF and put it into a crucible and bake it in a muffle furnace. The temperature is gradually raised and ground every half an hour until the temperature of the muffle furnace rises to 600°C and continues to dry for 15 hours. Dry in a vacuum oven at 150°C for 3 hours before use;
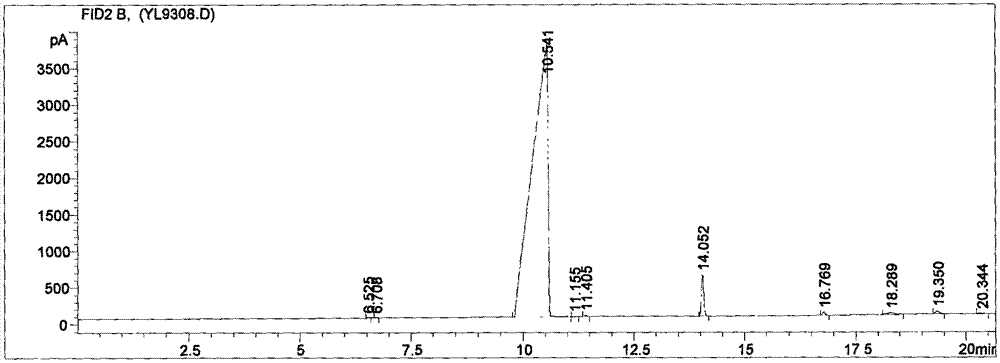
[0051] Step 2: Put 1.2g of SD-KF just pretreated and 0.03g of quaternary phosphonium salt II containing an alkyltriphenyl substituent into a 100mL three-necked flask connected with a condenser 1 , 1.6g p-chloronitrobenzene, 20mLDMSO, replace the air in the reaction bottle with nitrogen, then add it to reflux reaction under nitrogen protection for 6h, sample once per hour, and carry out gas chromatography detection;
[0052] ...
Embodiment 3
[0055] A quaternary phosphonium salt containing a monoalkyl triphenyl substituent and a preparation method thereof. The quaternary phosphonium salt containing an alkyl triphenyl substituent is denoted as II 2 , whose structural formula is:
[0056]
[0057] The quaternary phosphonium salt containing multiple alkyl triphenyl substituents is denoted as II 2 The concrete steps of the preparation method are:
[0058] Step 1, quaternary phosphonium halide I 2 preparation of
[0059] Quaternary Phosphonium Halide I 2 The structural formula is:
[0060]
[0061] Quaternary Phosphonium Halide I 2 Preparation: Add 7.86g (0.03mol) of triphenylphosphine and 20ml of acetonitrile into a 100ml three-necked flask equipped with a condenser tube, add 8mL (0.06mol) of bromobutane dropwise at constant pressure under nitrogen protection, reflux and stir for 10 hours, and react After completion, a uniform yellow oily liquid was obtained, which was cooled in the refrigerator. After the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com