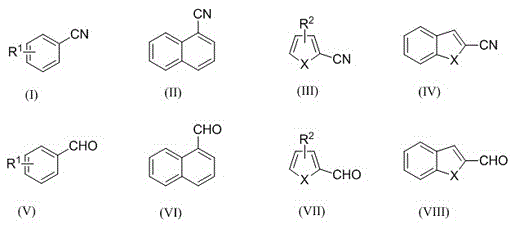
Electrochemical catalytic synthesis method of aromaticnitrile
A synthesis method and technology for aromatic nitrile, applied in the field of chemistry, can solve the problems of toxic cyanide source, environmental problems, strict control requirements, etc., and achieve the effects of reducing environmental cost, mild reaction conditions, simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
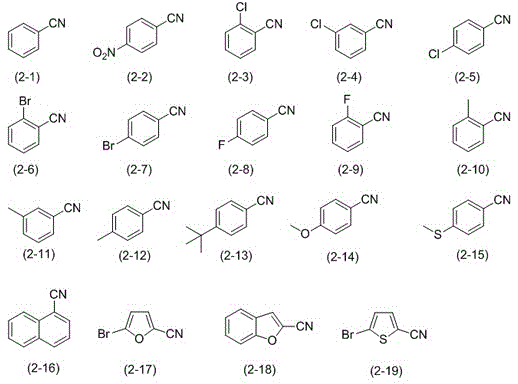
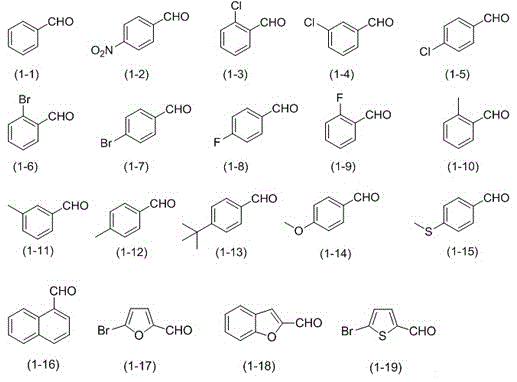
[0026] Embodiment 1: Preparation of benzonitrile (formula (2-1))
[0027] Add 0.1mol / L sodium perchlorate in acetonitrile solution (10mL), benzaldehyde (formula (1-1), 1mmol, 106mg), TEMPO (0.1mmol, 16mg), HMDS (2.5mmol, 404mg) into a 30ml beaker ) and acetic acid (2.5mmol, 150mg). 25°C, constant potential electrolysis at 1.5V, and the reaction ended after 15 hours. Add saturated sodium sulfite solution to the reaction solution and stir, then extract with dichloromethane, separate the organic layer, distill off the solvent under reduced pressure, and then perform column chromatography separation, and use a mixture of ethyl acetate / petroleum ether with a volume ratio of 1:200 as Eluent, collect the eluate containing the target compound, evaporate the solvent to obtain 82.4 mg of benzonitrile, and the separation yield is 80%.
Embodiment 2
[0028] Embodiment 2: Preparation of p-nitrobenzonitrile (formula (2-2))
[0029] Add 0.1mol / L sodium perchlorate in acetonitrile solution (10mL), p-nitrobenzaldehyde (formula (1-2), 1mmol, 151mg), TEMPO (0.1mmol, 16mg), HMDS (2.5 mmol, 404mg) and acetic acid (2.5mmol, 150mg). 25°C, constant potential electrolysis at 1.5V, and the reaction ended after 12 hours. Add saturated sodium sulfite solution to the reaction solution and stir, then extract with dichloromethane, separate the organic layer, distill off the solvent under reduced pressure, and then perform column chromatography separation, and use a mixture of ethyl acetate / petroleum ether with a volume ratio of 1:200 as Eluent, collect the eluate containing the target compound, evaporate the solvent to obtain 136.2 mg of p-nitrobenzonitrile, and the separation yield is 92%.
Embodiment 3
[0030] Embodiment 3: Preparation of p-nitrobenzonitrile (formula (2-2))
[0031] The reaction steps were the same as in Example 2, except that the voltage was changed to 1.0V, and the reaction was performed for 15 hours. Finally, 130.2 mg of p-nitrobenzonitrile was obtained, and the isolated yield was 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com