A kind of preparation method of lithium ion battery
A lithium-ion battery and electrolyte technology, which is applied in the manufacture of electrolyte batteries, secondary batteries, non-aqueous electrolyte batteries, etc., can solve problems such as increasing initial discharge capacity, affecting battery rate performance, and battery volume expansion, so as to reduce volume Expansion rate, improvement of battery cycle performance, and effects of stabilizing battery potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
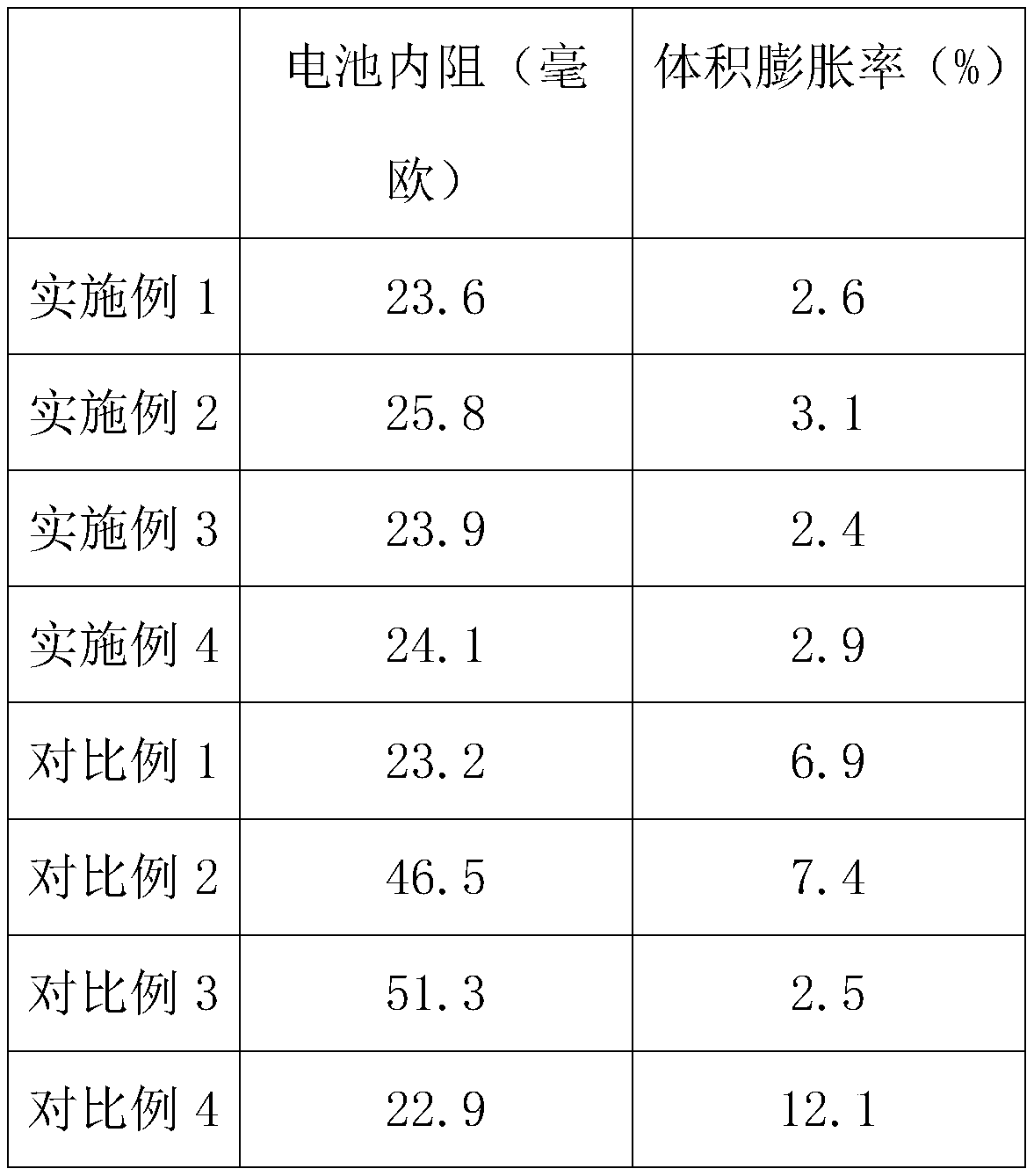
Embodiment 1
[0028] In the electrolyte in this embodiment, ethylene sulfate accounts for 0.5% of the total volume of the electrolyte, and the concentration of sodium hexafluorophosphate in the electrolyte is 0.001mol / L;
[0029] 1), inject electrolyte;
[0030] 2), move the lithium-ion battery after liquid injection into the glove box and charge it to 3.6V with a constant current of 0.02C, wherein the atmosphere in the glove box is nitrogen containing 10% by volume of carbon dioxide;
[0031] 3) Charge with a constant voltage of 3.6V until the charging current drops below 0.01C;
[0032] 4), between 3.55-3.65V, use a current of 0.02C to perform constant current charge and discharge cycles for 3 times;
[0033] 5), adjust the atmosphere in the glove box, reduce the volume percentage of carbon dioxide in the nitrogen to 3%;
[0034] 6), between 3.55-3.65V, use a current of 0.02C to perform constant current charge and discharge cycles for 3 times;
[0035] 7) Charge with a constant current...
Embodiment 2
[0038] In the electrolyte in this embodiment, ethylene sulfate accounts for 3% of the total volume of the electrolyte, and the concentration of sodium hexafluorophosphate in the electrolyte is 0.02mol / L;
[0039] 1), inject electrolyte;
[0040] 2), move the lithium-ion battery after liquid injection into the glove box and charge it to 3.6V with a constant current of 0.02C, wherein the atmosphere in the glove box is nitrogen containing 15% by volume of carbon dioxide;
[0041] 3) Charge with a constant voltage of 3.6V until the charging current drops below 0.01C;
[0042] 4), between 3.55-3.65V, use a current of 0.02C to perform constant current charge and discharge cycles for 3 times;
[0043] 5), adjust the atmosphere in the glove box, reduce the volume percentage of carbon dioxide in the nitrogen to 2%;
[0044] 6), between 3.55-3.65V, use a current of 0.02C to perform constant current charge and discharge cycles for 3 times;
[0045] 7) Charge with a constant current of...
Embodiment 3
[0048] In the electrolyte in this embodiment, ethylene sulfate accounts for 1% of the total volume of the electrolyte, and the concentration of sodium hexafluorophosphate in the electrolyte is 0.01mol / L;
[0049] 1), inject electrolyte;
[0050] 2), move the lithium-ion battery after liquid injection into the glove box and charge it to 3.6V with a constant current of 0.02C, wherein the atmosphere in the glove box is nitrogen containing 10% by volume of carbon dioxide;
[0051] 3) Charge with a constant voltage of 3.6V until the charging current drops below 0.01C;
[0052] 4), between 3.58-3.62V, use a current of 0.02C to perform constant current charge and discharge cycles for 3 times;
[0053] 5), adjust the atmosphere in the glove box, the volume percentage of carbon dioxide in the nitrogen is reduced to 0;
[0054] 6), between 3.58-3.62V, use a current of 0.02C to perform constant current charge and discharge cycles for 3 times;
[0055] 7) Charge with a constant current...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com