High nickel anode material, preparation method thereof and lithium ion battery
A cathode material, high nickel technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of poor battery safety and thermal stability, reduced battery electrochemical performance, damage to the crystal structure of the material surface, etc. Effects of chemical stability and safety, good electrochemical stability and safety, good capacity and cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
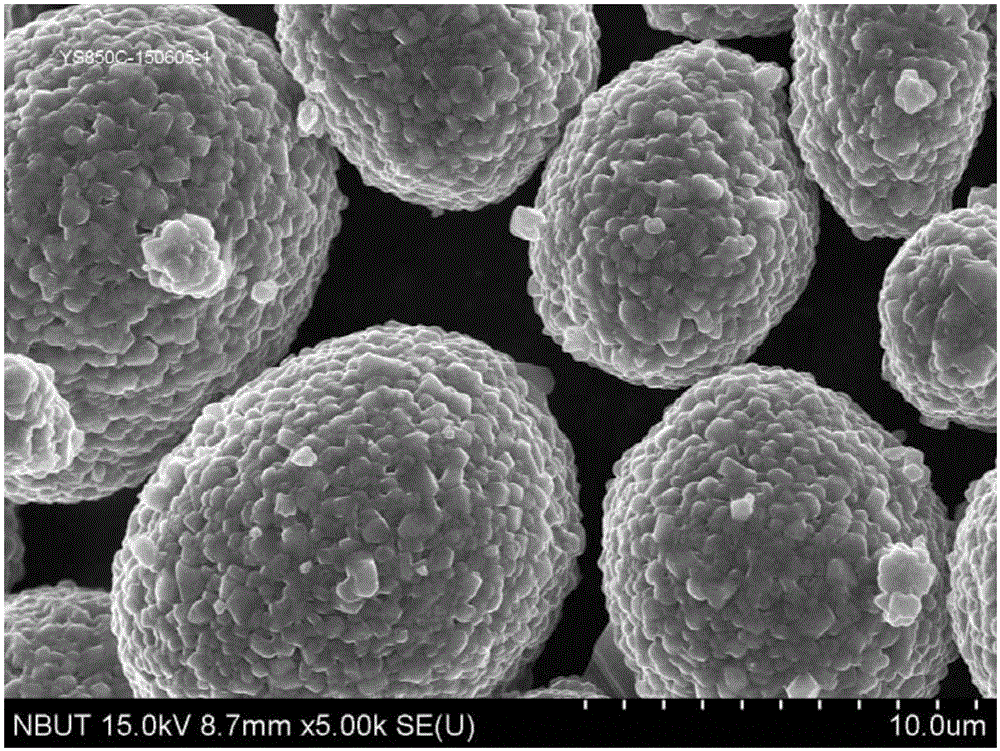
Image
Examples
preparation example Construction
[0062] The present invention provides a method for preparing the high-nickel positive electrode material described in the above technical solution, comprising:
[0063] The compound of nickel-cobalt, lithium compound and doping element compound are mixed and then calcined to obtain the matrix, and the compound of nickel-cobalt is the compound shown in formula I or the oxide obtained by oxidation of the compound shown in formula I:
[0064] Ni 1-x-y co x m y (OH) 2 Formula I,
[0065] In formula I, 0.05≤x≤0.30, 0.01≤y≤0.20,
[0066] M is one or more of Mn, Al, Ti, Mg, Zr, Ca, Zn, Sr, La and B;
[0067] The doping elements in the doping element compound are Al, Ti, Mg, Zr, Ca, Zn, B, F, V, Sr, Ba, Y, Nd, Cs, W, Mo, Ru, Rd and lanthanide one or more of the elements;
[0068] The amount of the nickel-cobalt compound and the lithium compound makes the ratio of the total moles of Ni, Co and M to the moles of Li be 1:(0.9~1.15);
[0069] mixing the substrate and the coating a...
Embodiment 1
[0116] Prepare nickel-cobalt-aluminum compound according to the method described in Comparative Example 1 of the present invention;
[0117] The nickel-cobalt-aluminum compound obtained above is uniformly mixed with lithium hydroxide and lanthanum nitrate for 1.5 h, and the amount of the nickel-cobalt-aluminum compound and lithium hydroxide is such that the ratio of the total moles of Ni, Co and Al to the moles of Li is 1 : 1.06, the addition of lanthanum nitrate is 0.5% of the mass of the above-mentioned nickel-cobalt-aluminum compound, the mixture obtained above is calcined at 800° C. for 15 hours in an oxygen atmosphere, and then crushed and sieved to obtain a doped product;
[0118] After stirring the above-mentioned doped product in deionized water at 60° C. for 0.5 h, an aluminum nitrate solution with a mass concentration of 130 g / L was added dropwise thereto, and the quality of the deionized water was 1.5 times the mass of the above-mentioned doped product. The quality ...
Embodiment 2
[0124] Nickel sulfate solution, cobalt sulfate solution and manganese chloride solution are mixed homogeneously, obtain mixed solution, the consumption of described nickel sulfate solution, cobalt sulfate solution and manganese chloride solution makes the mol ratio of nickel, cobalt and manganese be 0.8:0.1: 0.1, the total metal ion concentration in the mixed solution is 3mol / L;
[0125] The ammonia solution that is 15% by mass percentage content, the NaOH solution that concentration is 3mol / L and described mixed solution flow into the reaction kettle concurrently, the addition speed of described mixed solution is 15mL / min, the addition speed of ammonia solution is 2mL / min, the addition rate of NaOH solution is 4mL / min, heating and stirring at 50°C for precipitation reaction, washing the obtained reaction product and drying at 100°C to obtain Ni 0.8 co 0.1 mn 0.1 (OH) 2 Nickel-cobalt-manganese compounds;
[0126] The nickel-cobalt-manganese compound obtained above is unifo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com