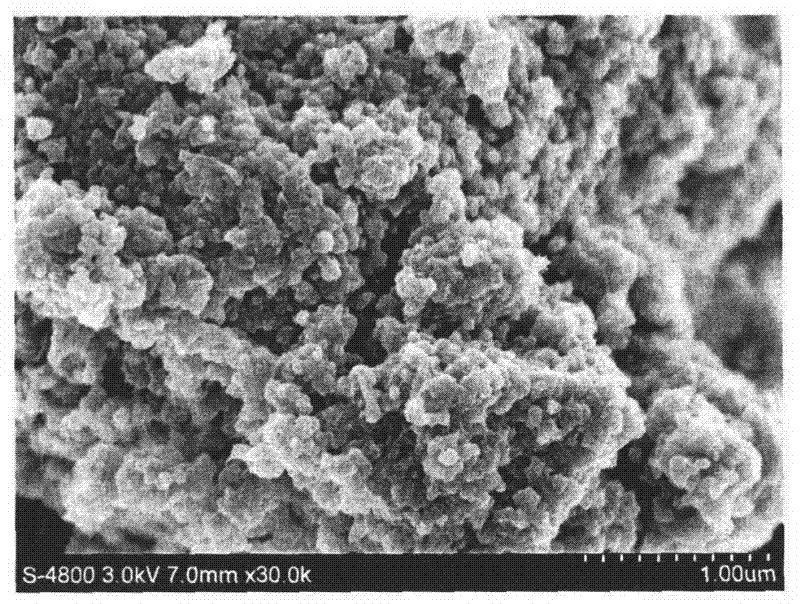
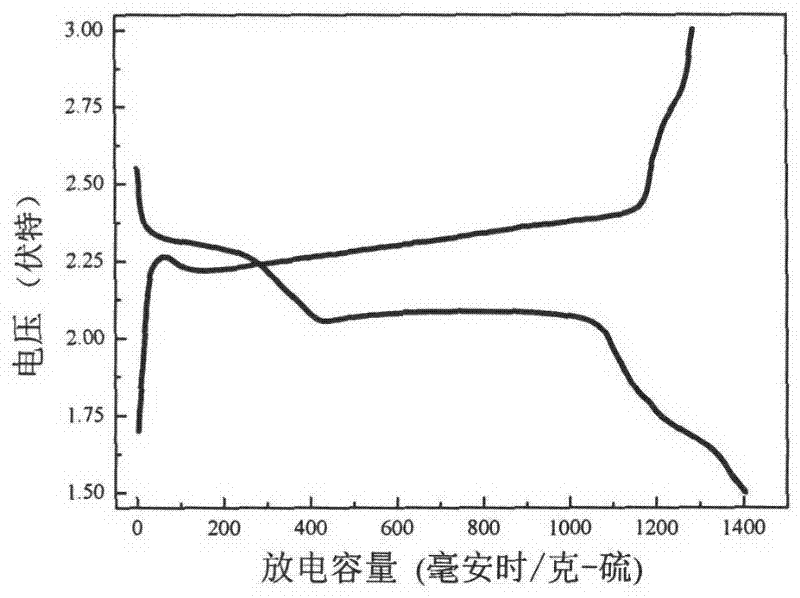
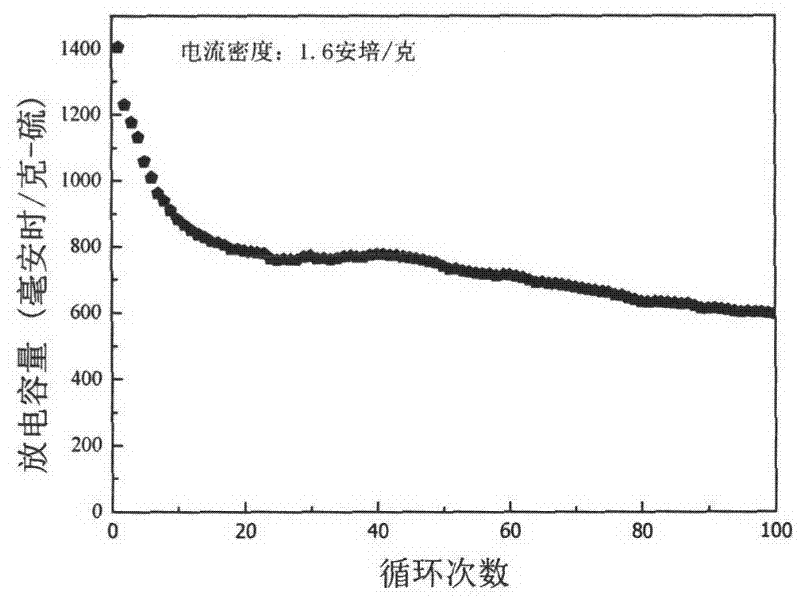
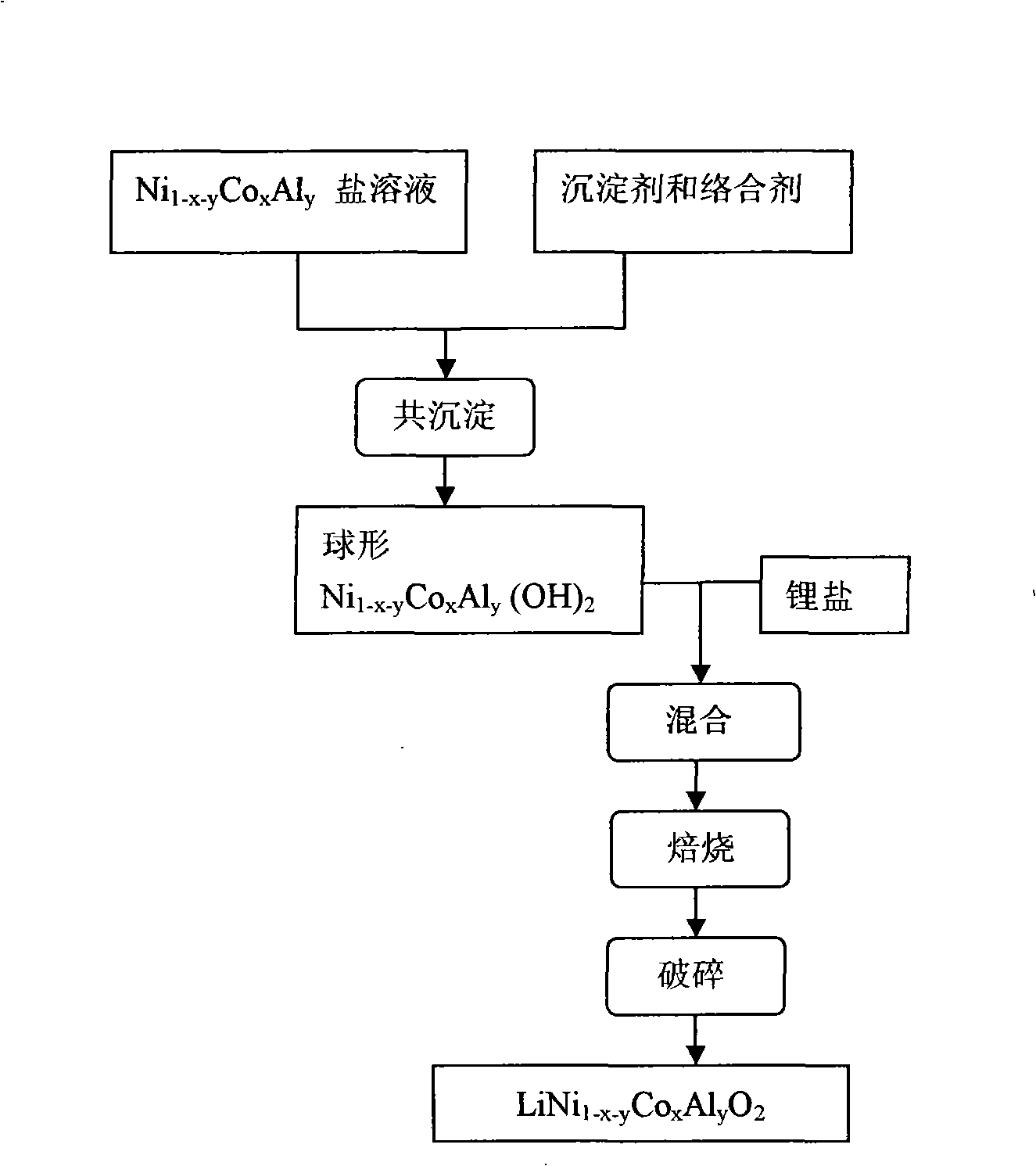
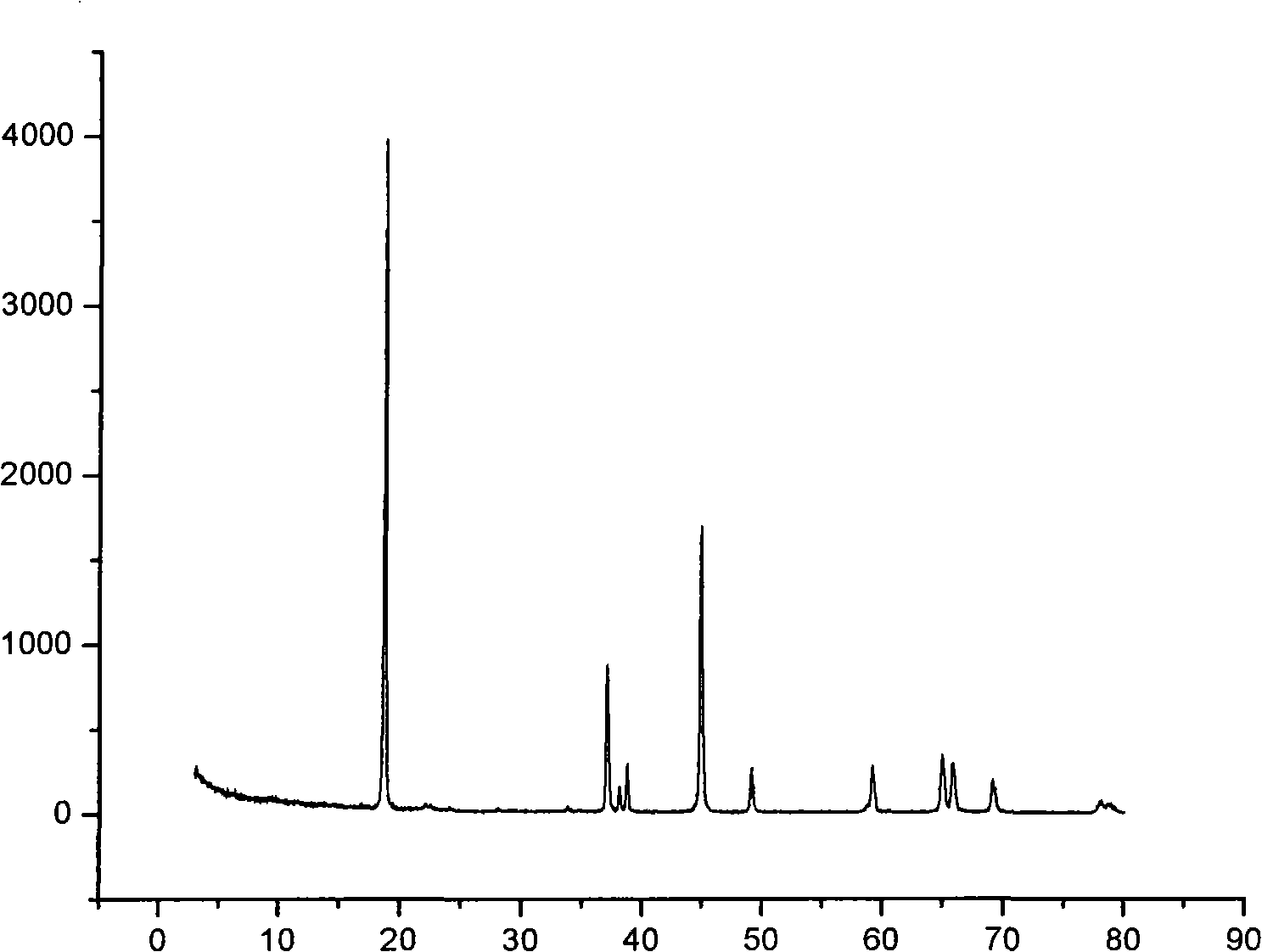
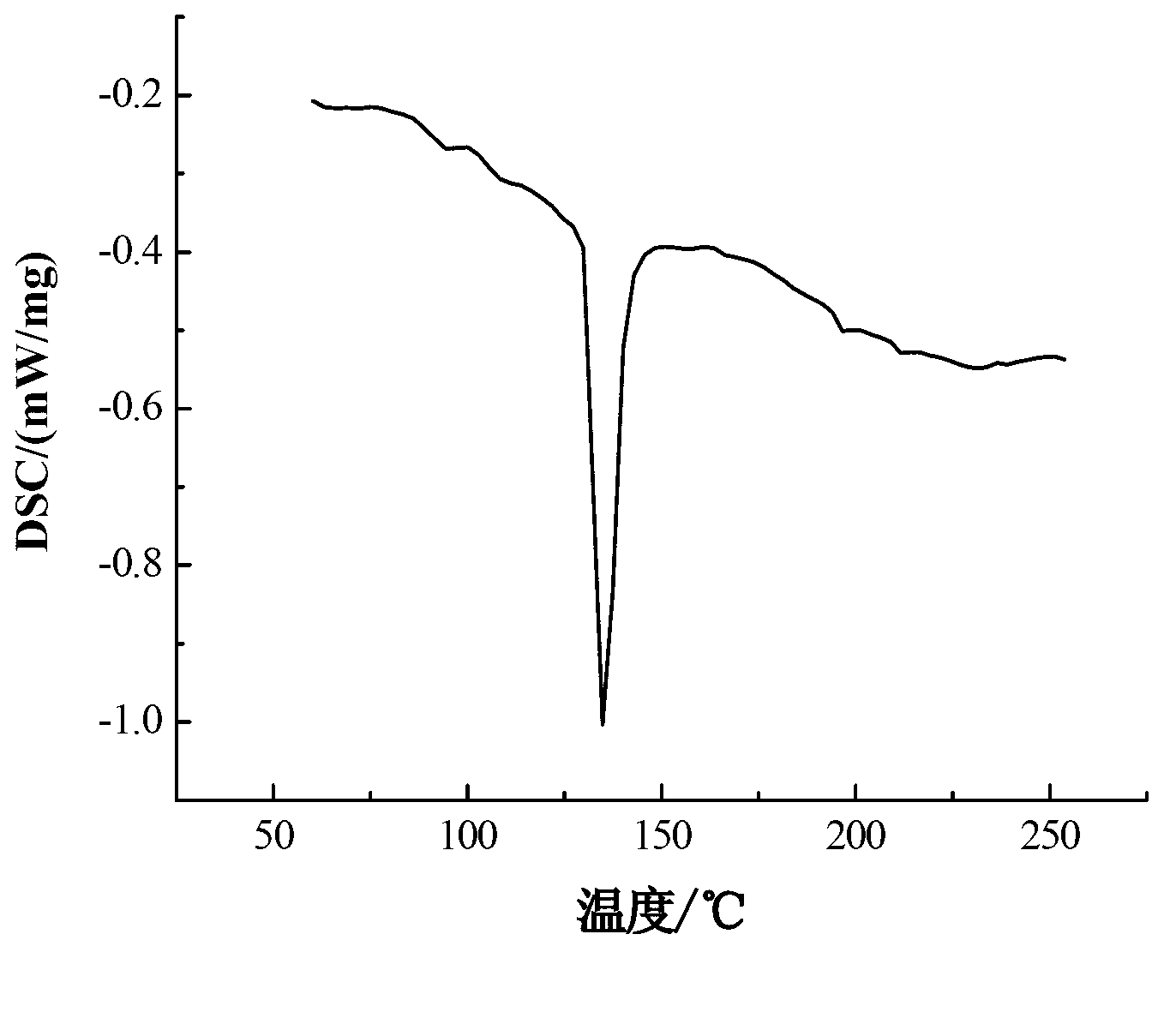
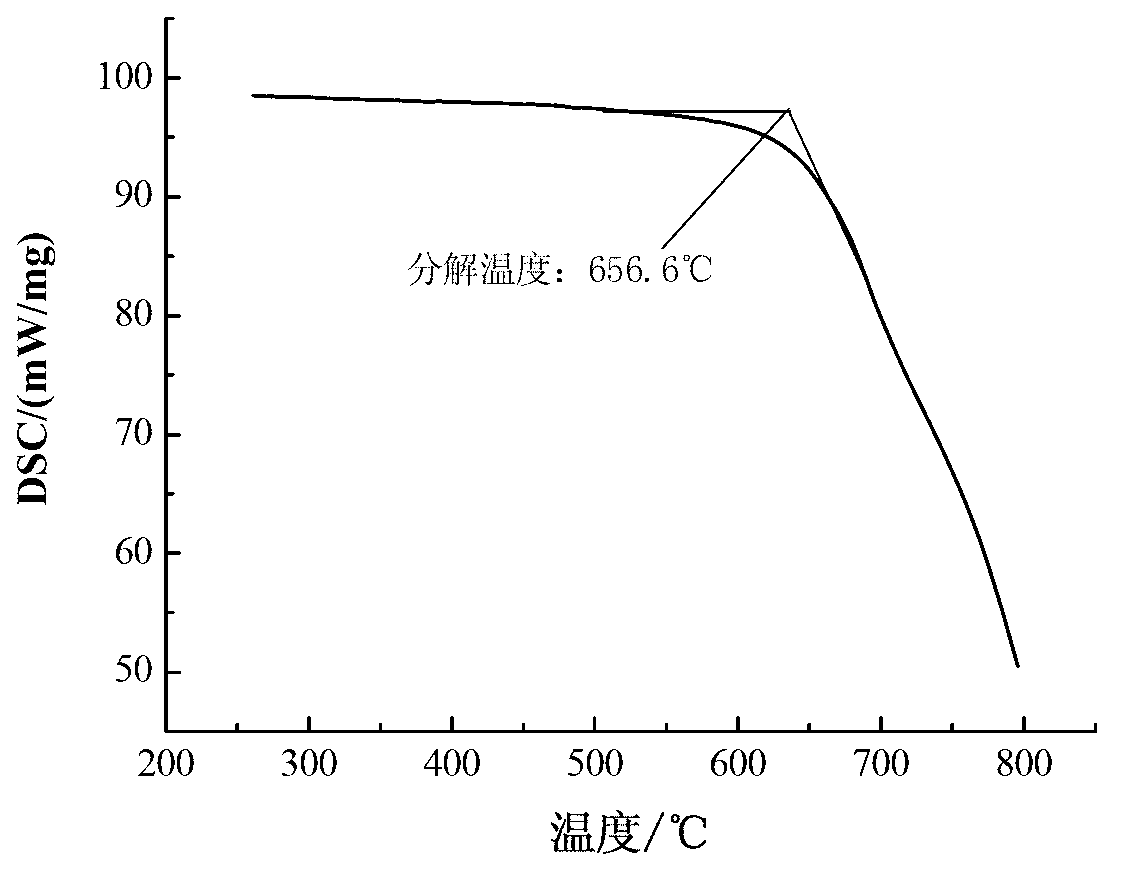
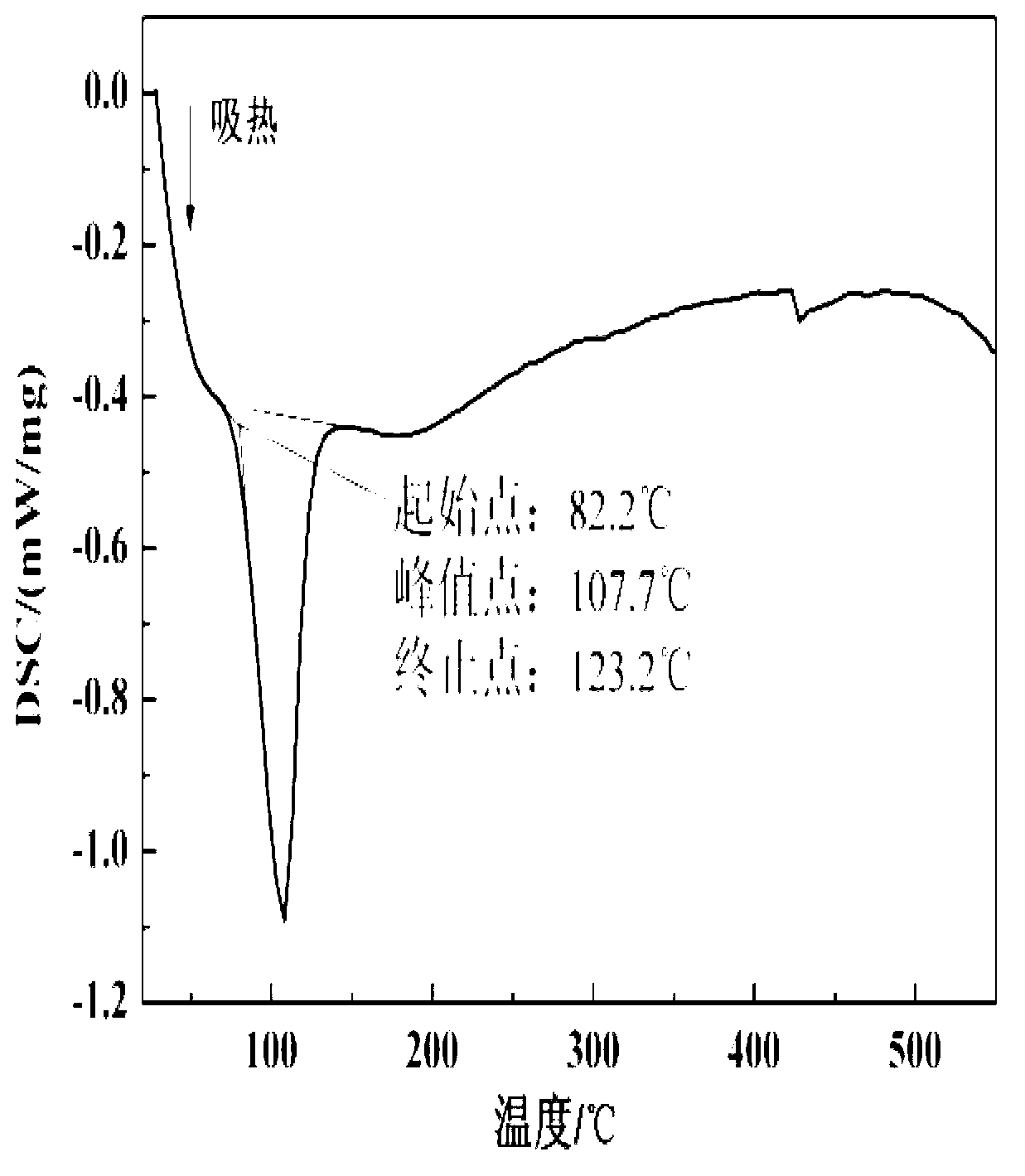
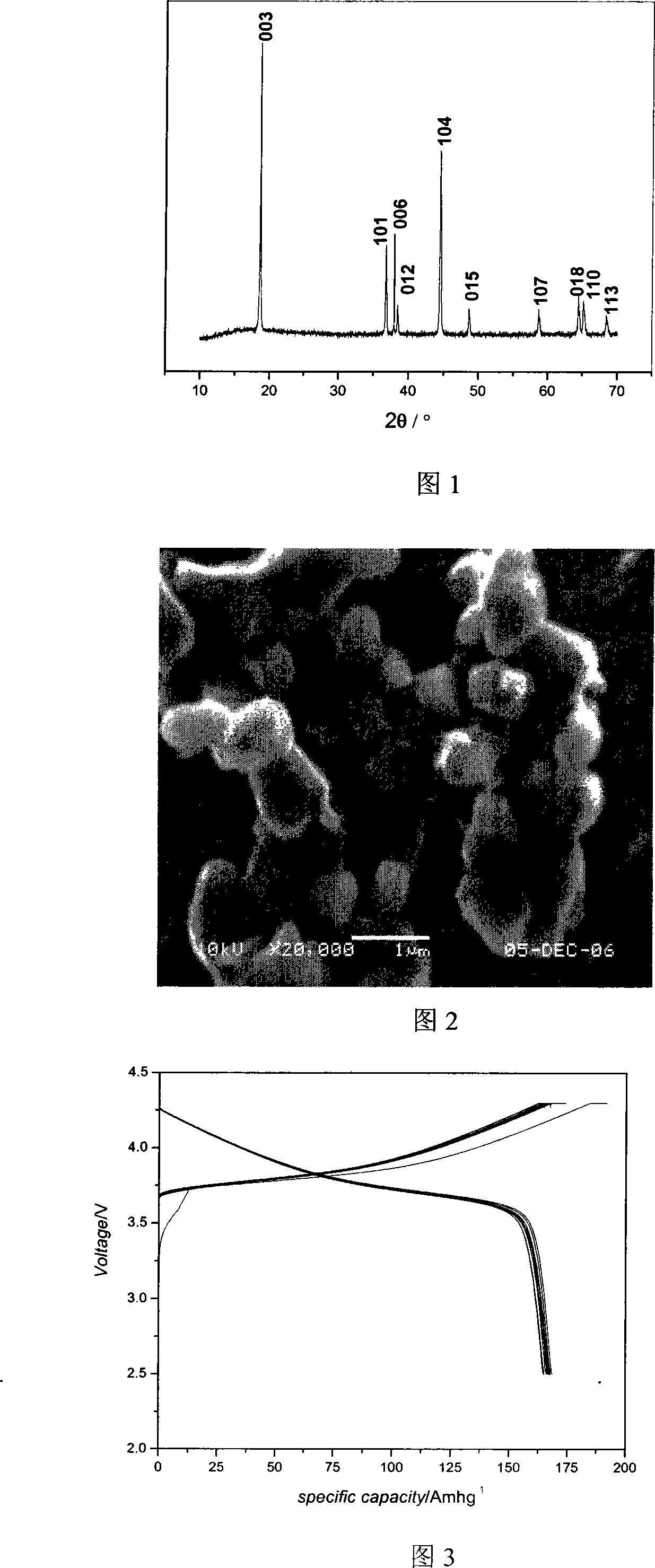
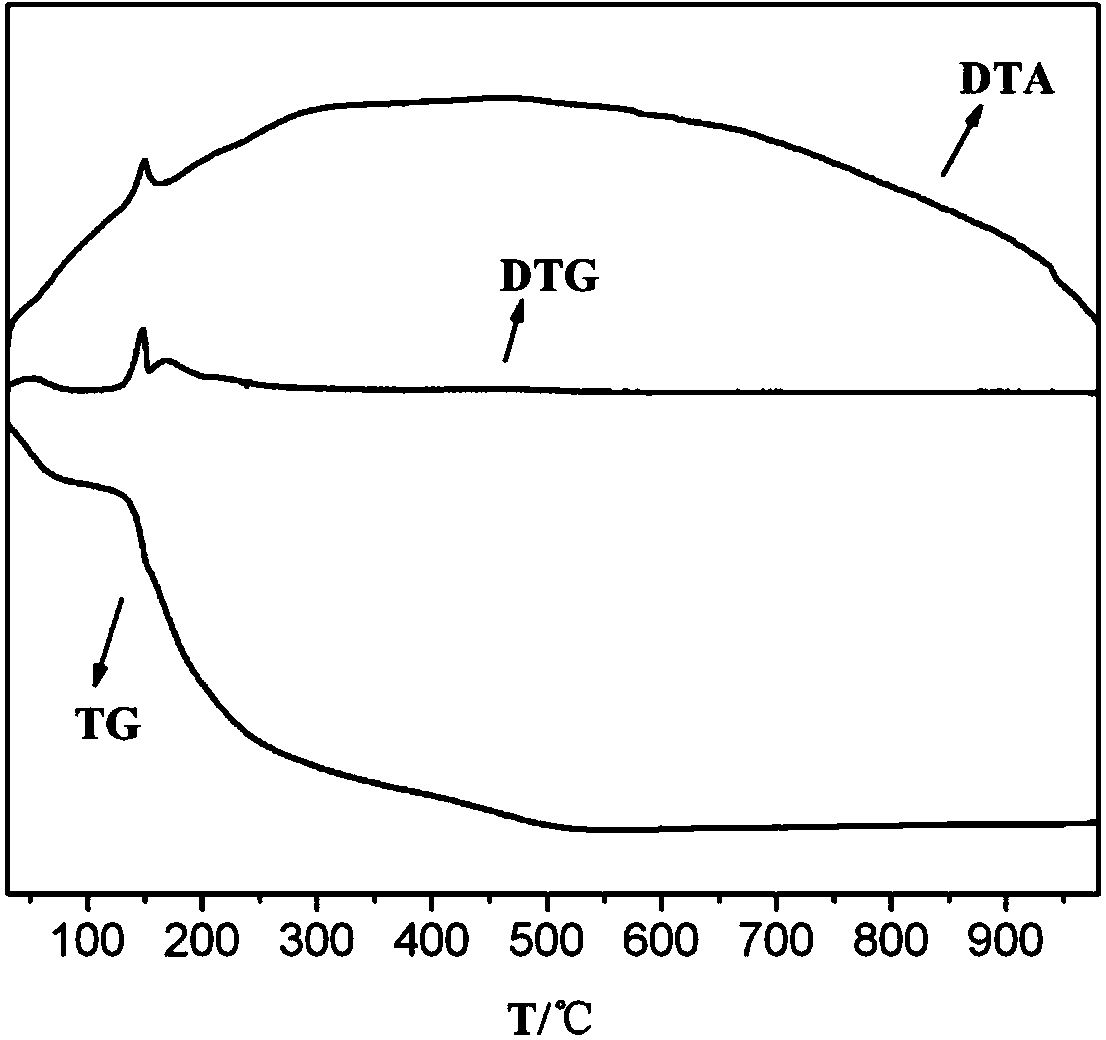
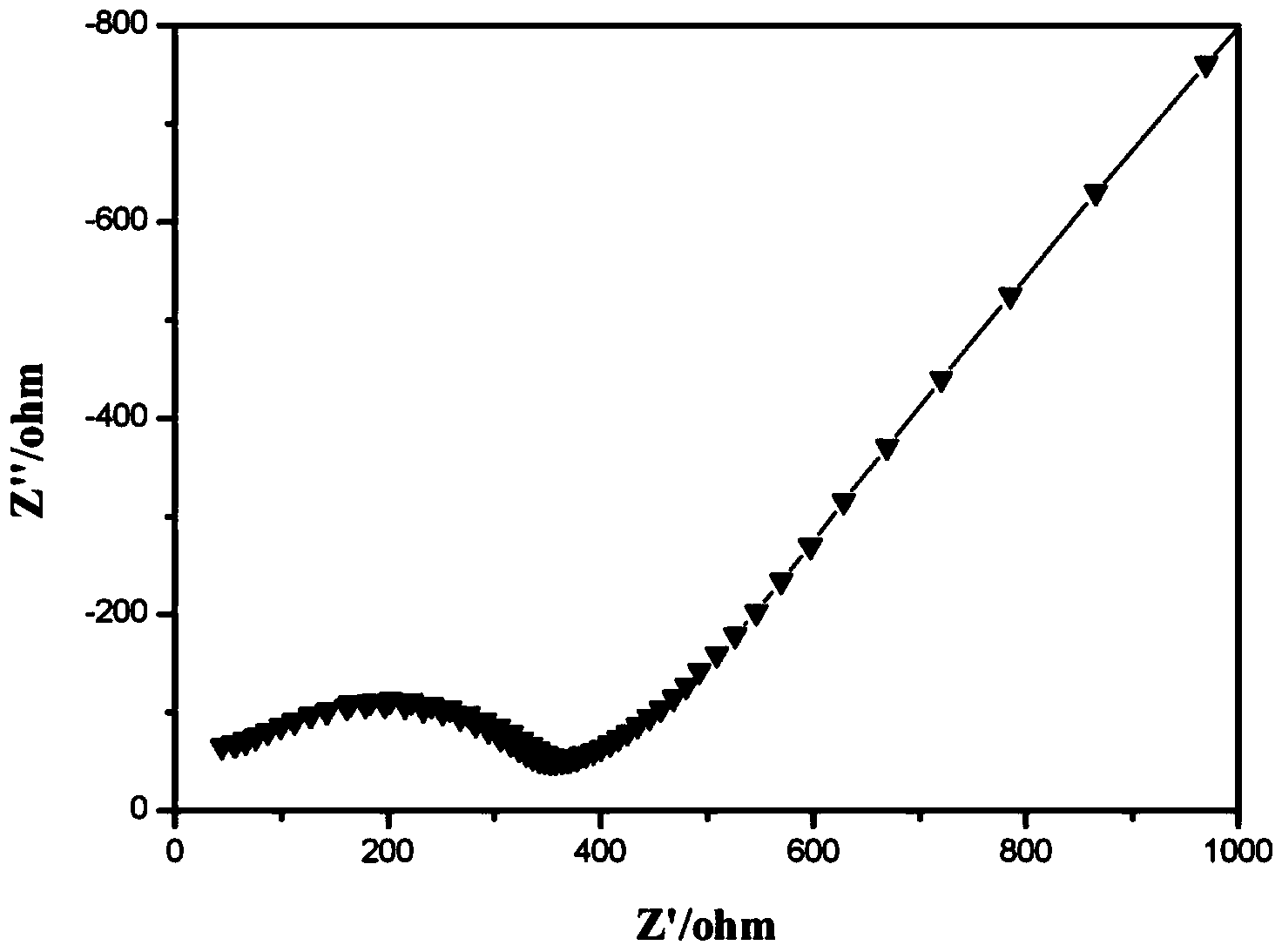
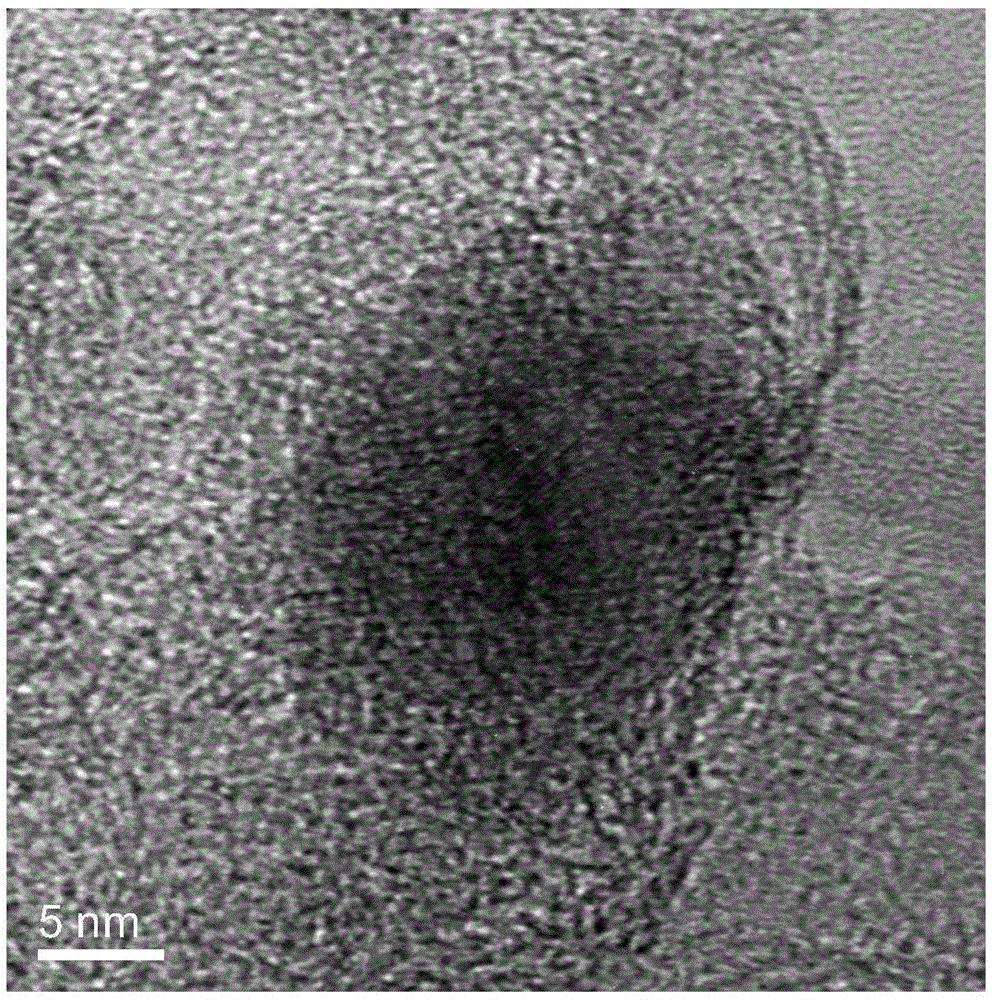
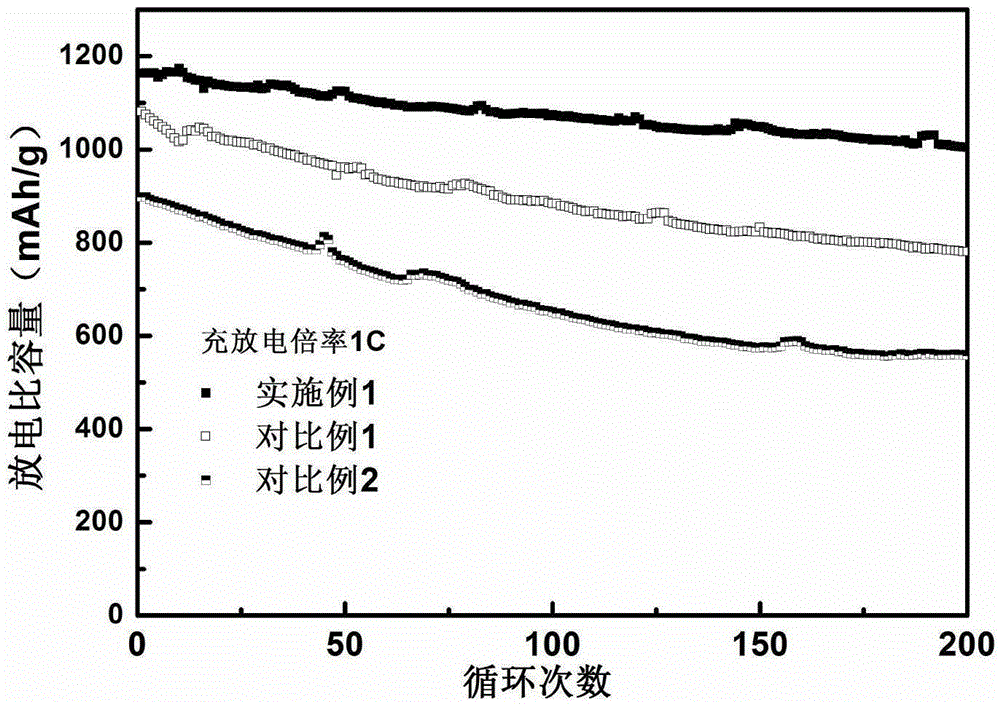
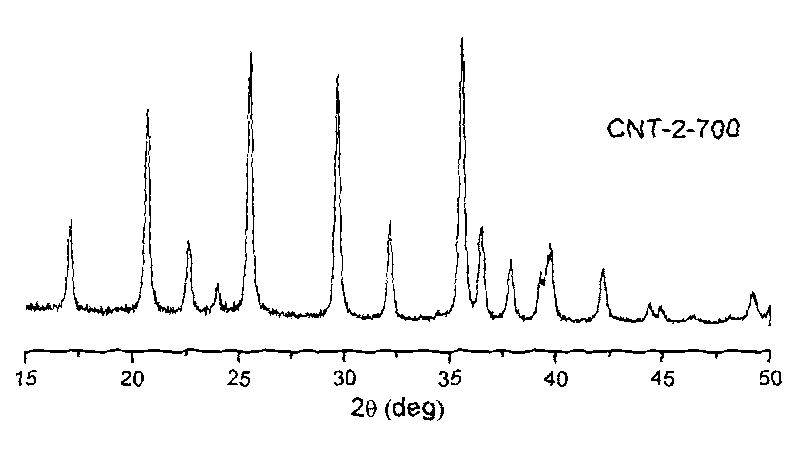
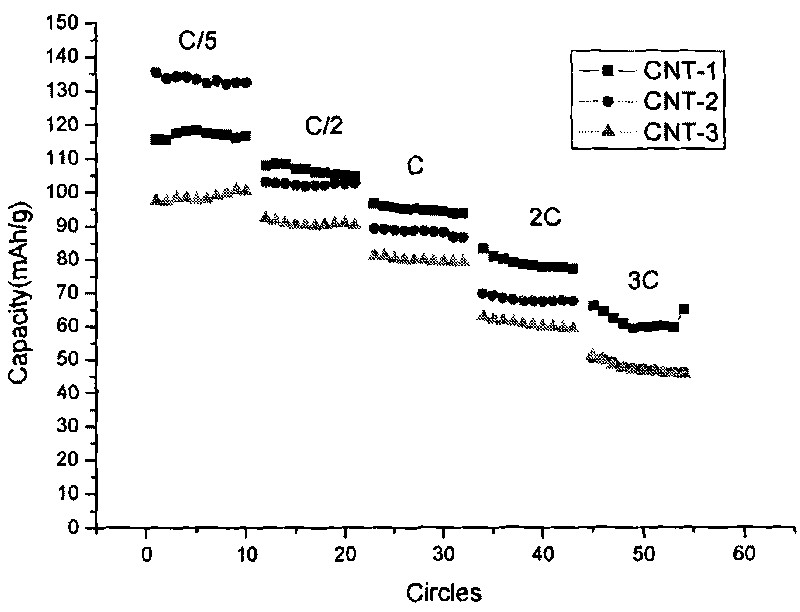
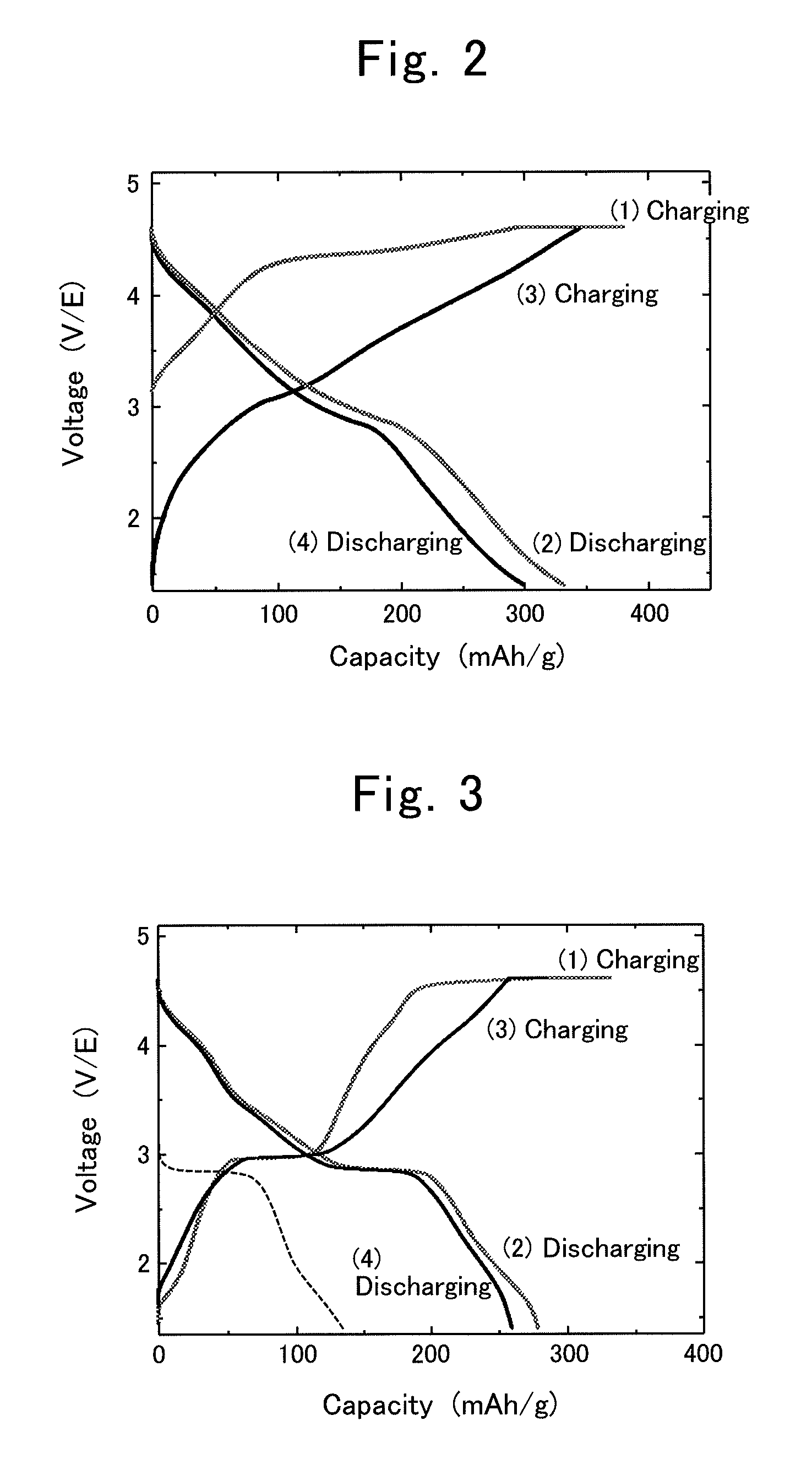
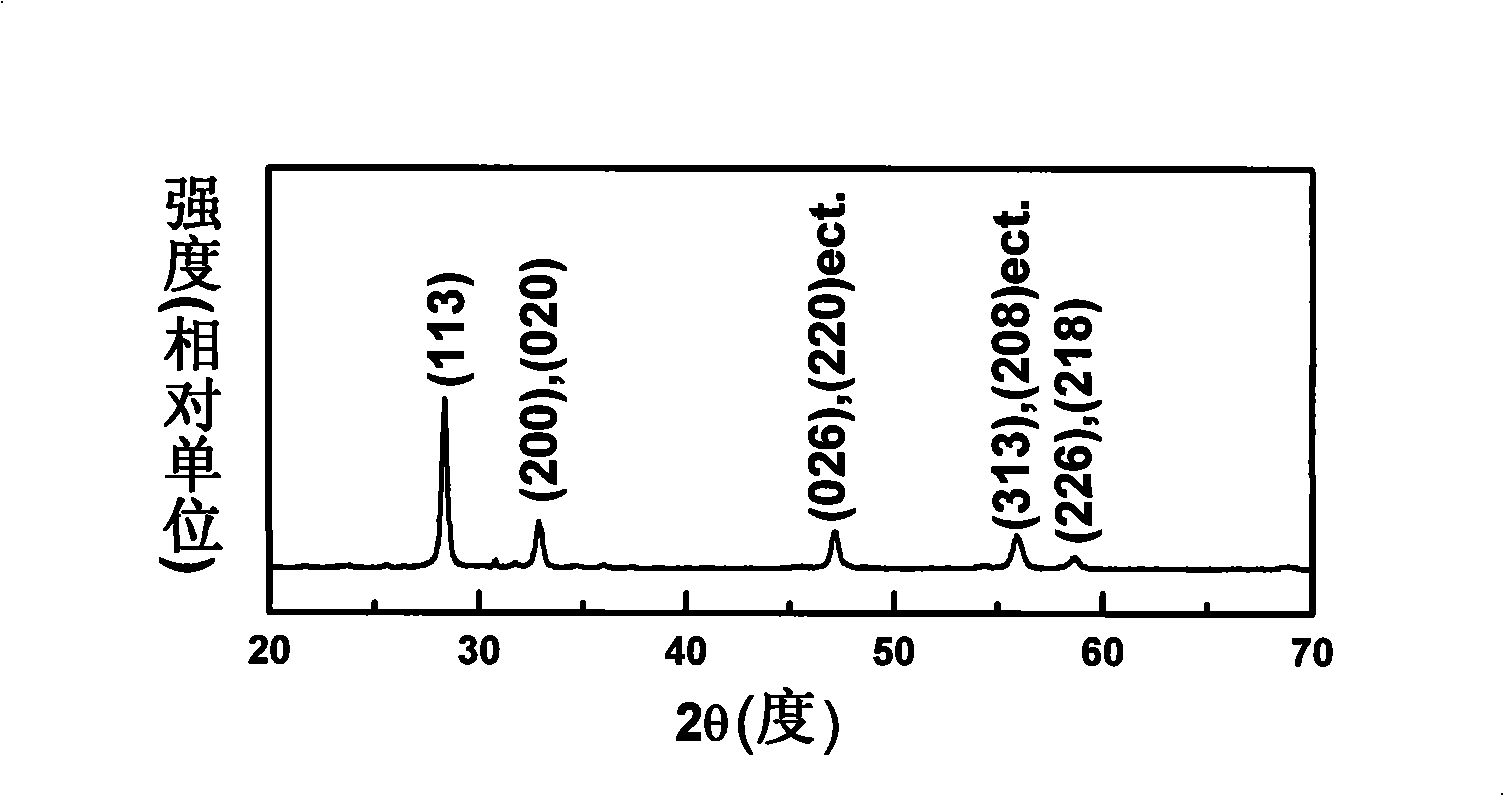
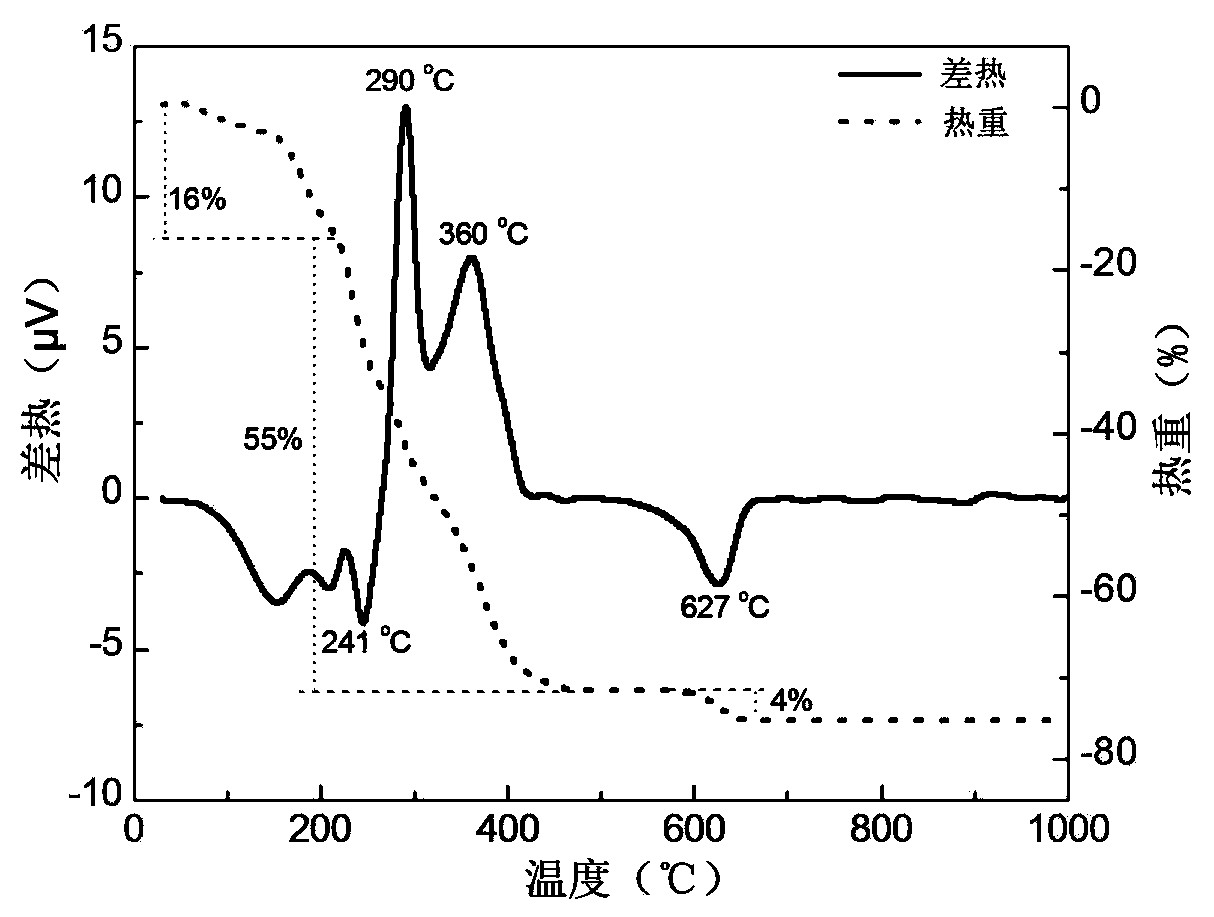
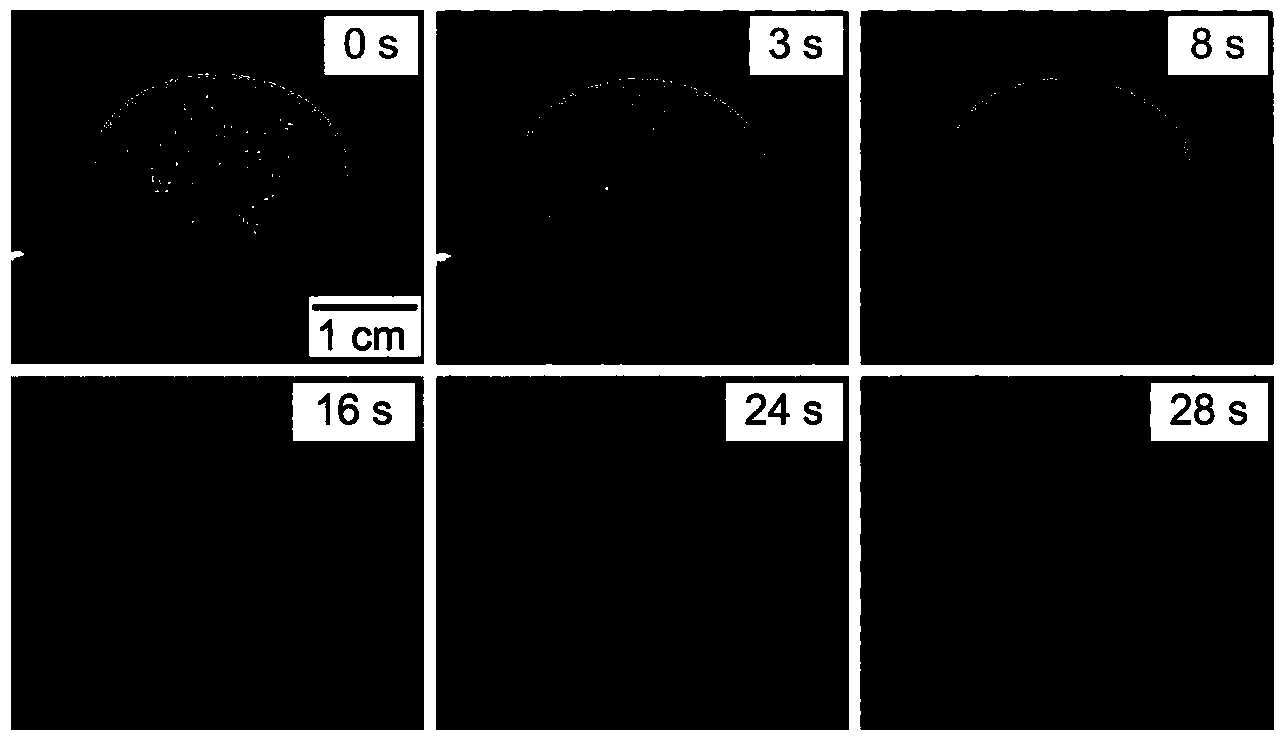
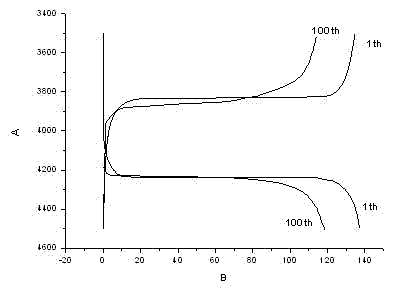
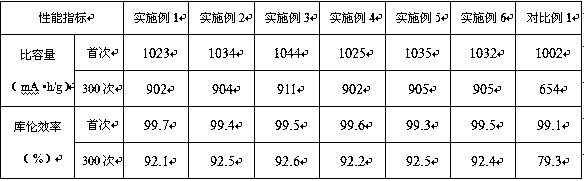
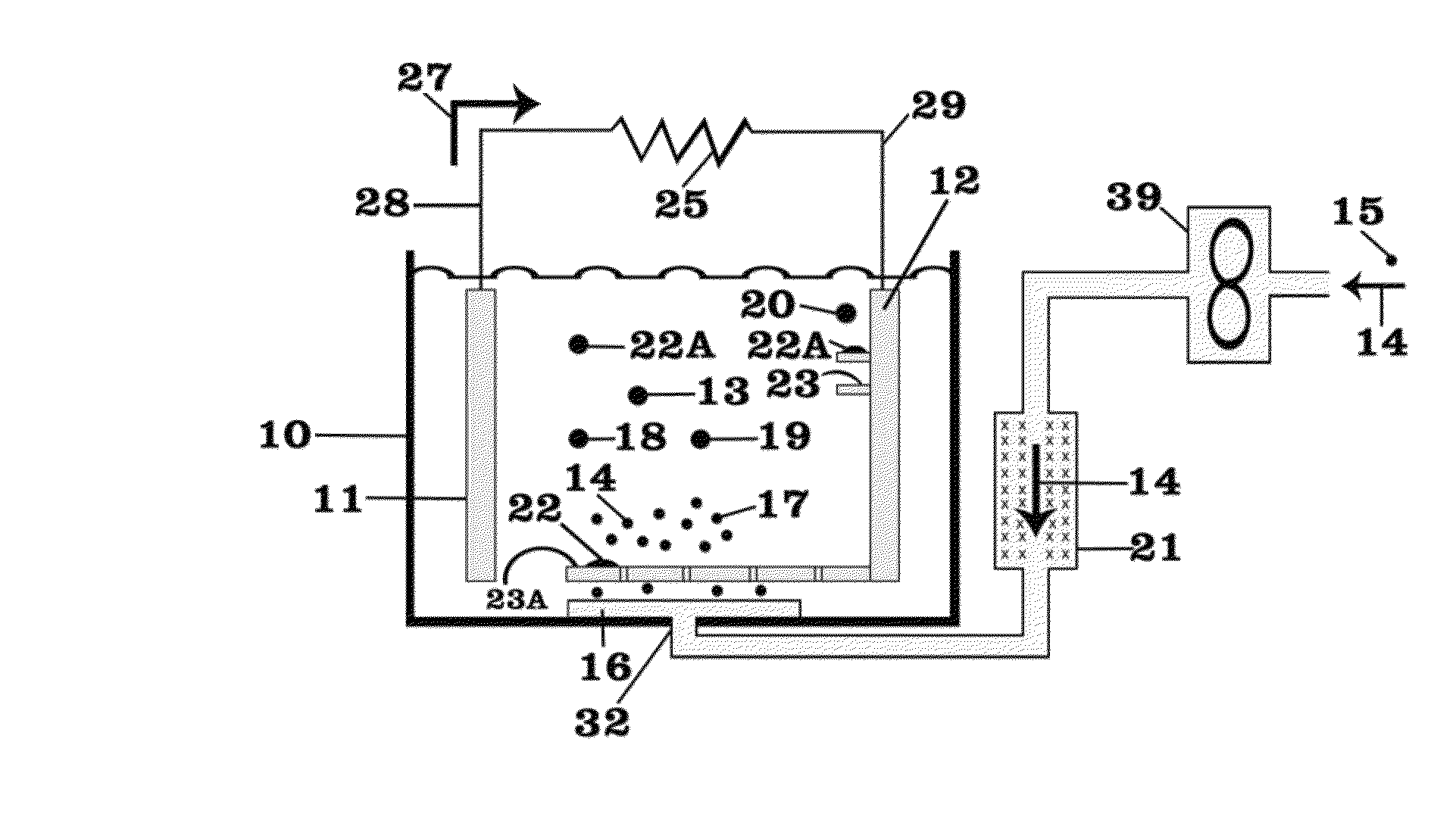
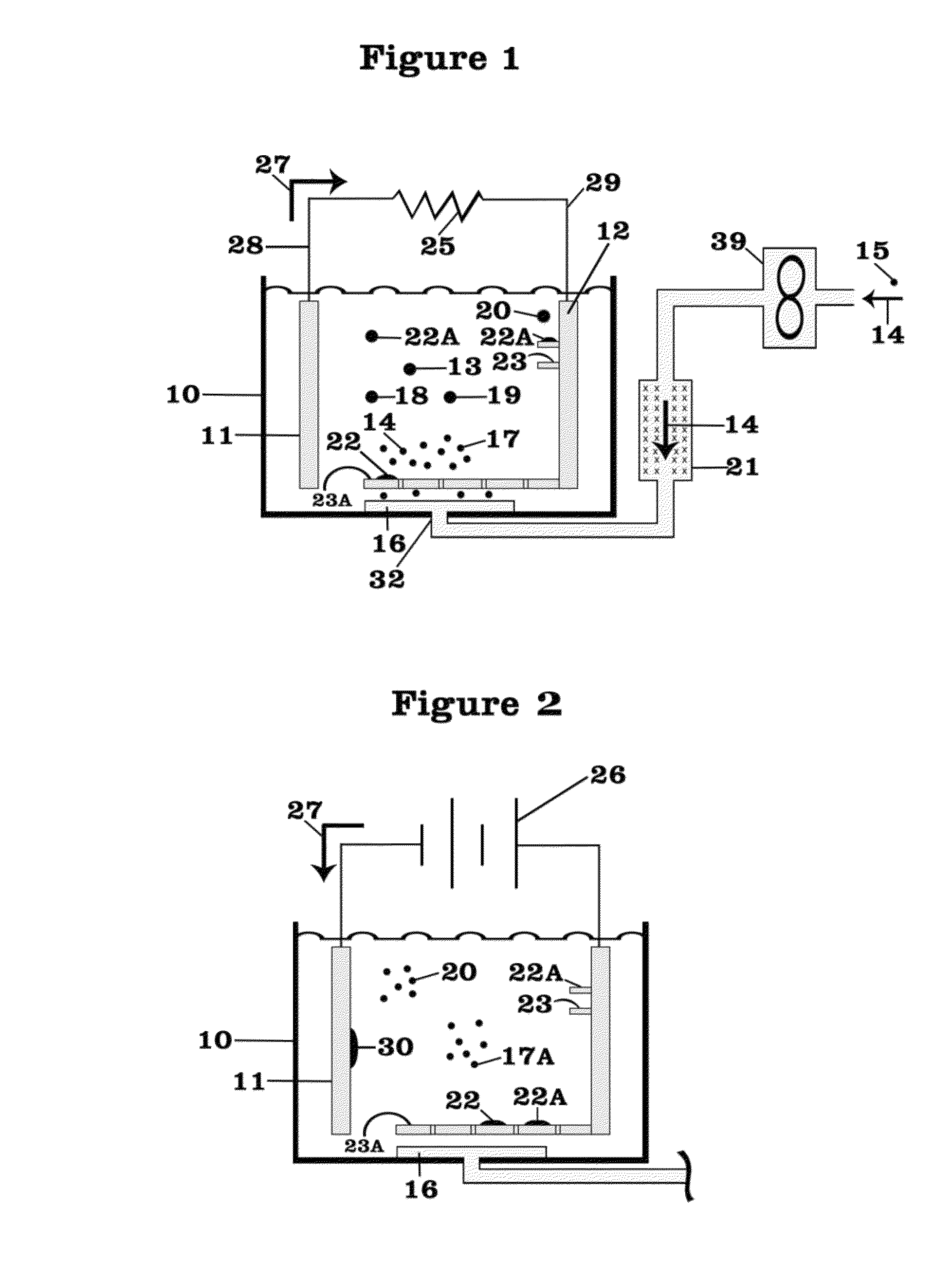
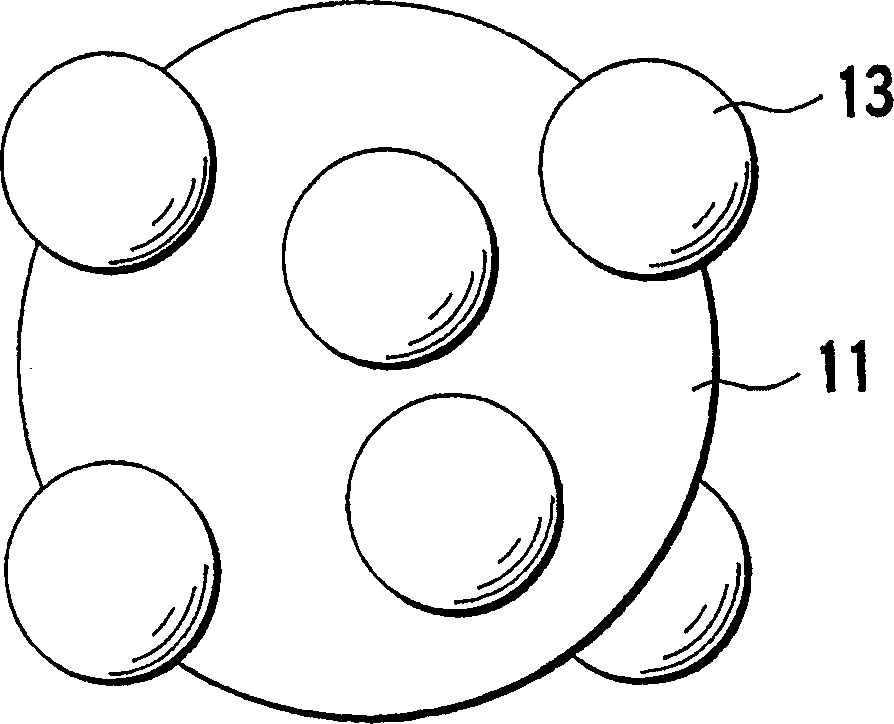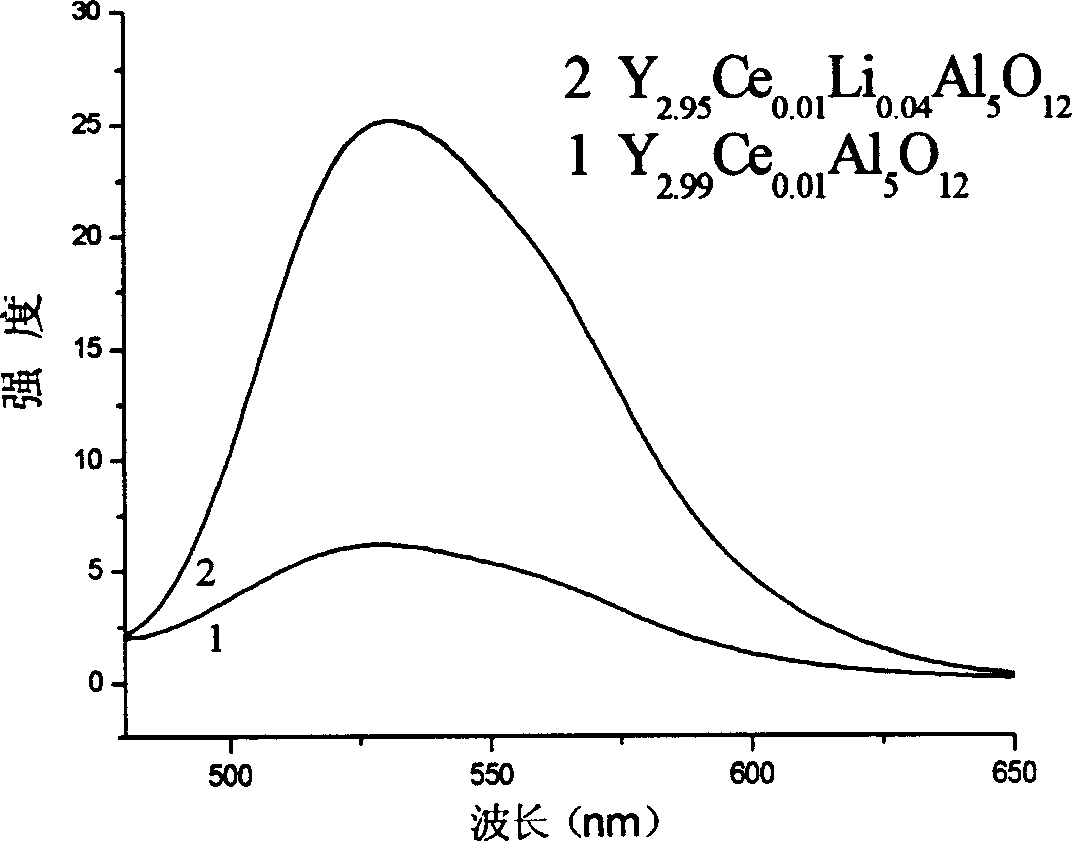Patents
Literature
530 results about "Lithium nitrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
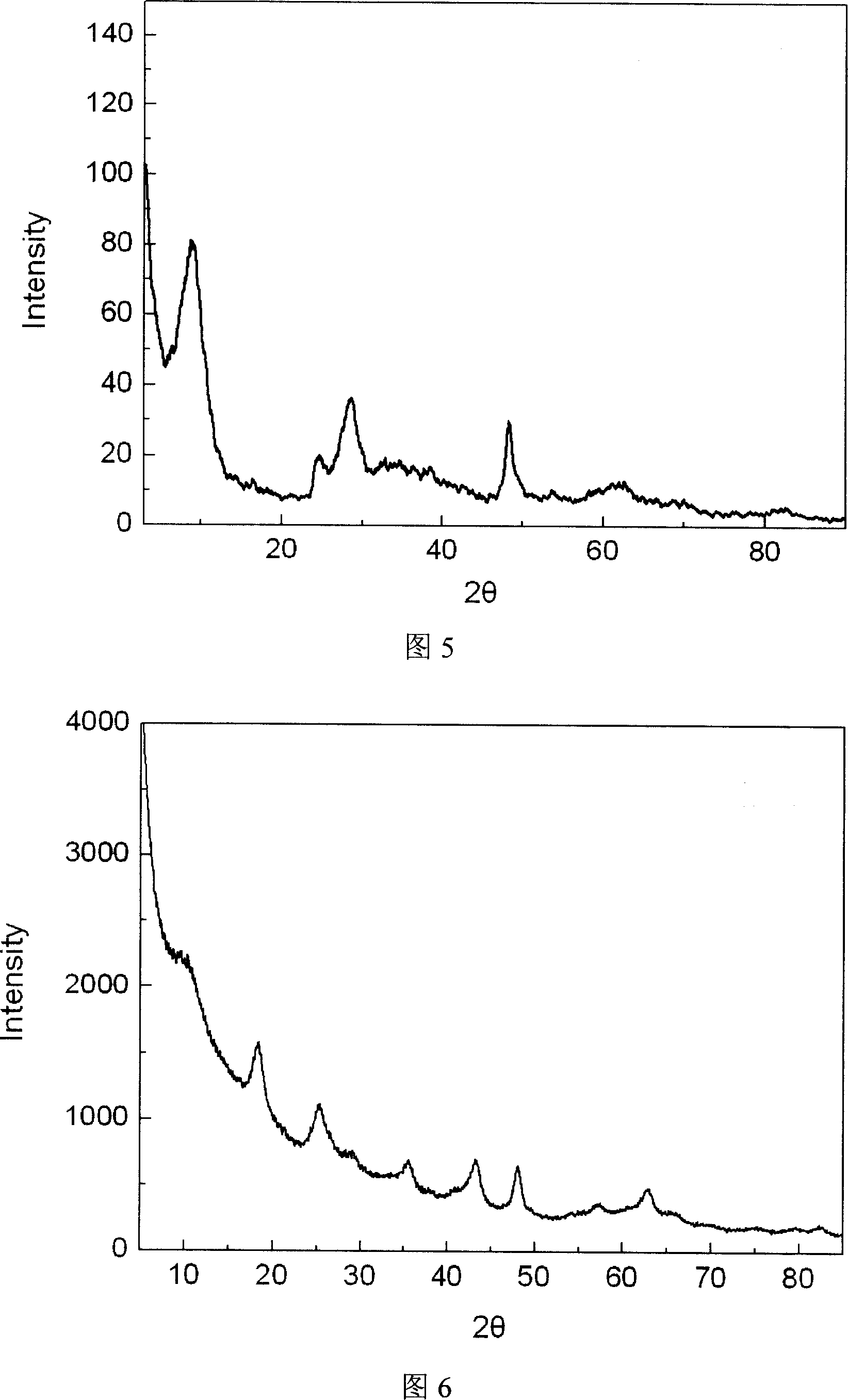
Lithium nitrate is an inorganic compound with the formula LiNO₃. It is the lithium salt of nitric acid (an alkali metal nitrate). The salt is deliquescent, absorbing water to form the hydrated form, lithium nitrate trihydrate. Its eutectics are of interest for heat transfer fluids.
Lithium battery with polymer-coated sulfur/carbon composite material as anode
InactiveCN102447113AImprove cycle performanceImprove conductivityCell electrodesSecondary cellsCarbon compositesMass ratio
The invention relates to a lithium battery with a polymer-coated sulfur / carbon composite material as an anode. According to the invention, sublimed sulfur or sulfur powder and a conductive carbon material are mixed according to a mass ratio of 3:7-8:2; the mixture is subject to ball milling, such that a sulfur / carbon composite material is obtained; the composite material is dispersed in a solution, and a polymer monomer is added to the solution; under a low temperature and the protection of inert gas, an oxidizing agent is added for initiating polymerization; the material is centrifuged, washed, and dried; the obtained polymer-coated elemental sulfur / carbon composite material, acetylene black and PTFE are mixed; a dispersant is added to the mixture, and the mixture is sufficiently mixed by stirring; the mixture is rolled into a sheet, and is vacuum-dried under a temperature of 55 DEG C, such that an electrode sheet is obtained. The prepared electrode sheet is adopted as an anode, metal lithium is adopted as a cathode, and a solvent type organic solution system containing 0.2mol / L of a waterless lithium nitrate additive is adopted as electrolyte, and a battery is assembled. With the electrode material, the assembled lithium battery is advantaged in high specific capacity, good circulation stability, and excellent heavy-current charge / discharge performances. The preparation method is advantaged in simple process, low cost, and good repeatability.
Owner:NANKAI UNIV
Spherical aluminum-doped nickel cobalt lithium for lithium ion battery and its making method
ActiveCN101262061AImprove liquidityImprove charge and discharge cycle stabilityElectrode manufacturing processesLithium compoundsDischarge efficiencyHigh rate
The invention discloses a preparation method of a spherical doped Al-Ni lithium cobalt oxide for lithium-ion battery. The preparation steps are that: first, sulfate, nitrate or chlorate of Al-Ni-Co react with strong alkali that is added with complex agent in liquid phase; the pH value, the temperature and the feeding speed of the reaction solution are controlled so as to produce a spherical precursor of Al-Ni-Co hydroxide; then the spherical precursor of Al-Ni-Co hydroxide is dried and evenly mixed with lithium hydroxide, lithium nitrate or lithium carbonate and dried; the obtained mixture is roasted into a spherical doped Al-Ni lithium cobalt oxide. The spherical doped Al-Ni lithium cobalt oxide has comparatively high tap density and remarkable cycle stability in the process of high-rate charge / discharge cycle, which improves over charge performance of Ni-Co substance and first obviously enhances charge / discharge efficiency; in addition, the preparation method of the spherical doped Al-Ni lithium cobalt oxide has the advantages of being simple, controllable and suitable for industrialized production with low energy consumption, high efficiency, short reaction time and low cost.
Owner:成都巴莫科技有限责任公司
Mixed molten salt as heat transfer and storage medium low in melting point
The invention relates to a formula of a mixed molten salt for medium-high temperature heat transfer and storage, and belongs to the physical heat transfer and energy storage technology in innovative and high technologies. The mixed molten salt comprises components in a ratio as follows: 10wt% of calcium nitrate, 60-70 wt% of potassium nitrate, 10-20 wt% sodium nitrate and 10wt% of sodium nitrite; the melting point of the mixed molten salt is about 130 DEG C, which is reduced by nearly 90 DEG C relative to solar salt and is reduced by about 15 DEG C relative to Hitec salt; and the thermal decomposition temperature thereof reaches above 650 DEG C. Sodium nitrate in the molten salt is changed into lithium nitrate, and the specific component ratio is as follows: 18-20wt% of calcium nitrate, 50-55 wt% of potassium nitrate, 9-10 wt% sodium nitrate and 18-20wt% of sodium lithium nitrate; after the component ratio is changed, the melting point of the mixed molten salt is about 90 DEG C, which is reduced by nearly 130 DEG C relative to the solar salt and is reduced by about 50 DEG C relative to the Hitec salt; and the thermal decomposition temperature thereof reaches above 600 DEG C. 10wt% of sodium carbonate is added continuously, then the melting point is raised to about 110 DEG C, while the decomposition temperature is raised by nearly 20 DEG C.
Owner:河北井矿新能源科技有限公司
Coprecipitation-combustion synthesis method for lithium nickel cobalt manganate
InactiveCN101215011AGood chemical stabilityEliminate ingredient lossCell electrodesNickel compoundsSynthesis methodsManganate
The invention discloses a process of coprecipitation-combustion synthesis of nickel cobalt manganese lithium carbonate. (1) Utilizing acetate or nitrate of nickel, cobalt, and manganese as transition metal source and ammonia as complexing agent and utilizing H2C2O4, (NH4)3C2O4, (NH4)2CO3 or NH4HCO3 as precipitator, compound carbonate contained Ni-Co-Mn or oxalate precursors is synthesized by coprecipitation method. (2) Directly drying the compound carbonate containing Ni-Co-Mn or the suspension liquid of the oxalate and adding lithium nitrate or lithium acetate or a small quantity of water or ethanol to adjust into rheological phase. (3) Laying the materials in rheological phase in an electric stove to perform burning synthesis reaction, wherein the electric stove heats the materials in rheological phase at temperature of 400-600DEG C and then keeps constant temperature. (4) Temper drawing the reaction product with temperature of 600-1200DEG C, and anode active materials of lithium ion battery LiNixCoyMn1-x-yO2 is obtained. The invention has the advantages of simple technique, easy operation, saving water and energy and environment-friendliness, further, the synthetic material is provided with the shape of sphere or near-sphere, high specific capacity and fine cycle performance.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Preparation method of NASICON-type lithium ion solid electrolyte
ActiveCN103825052AOvercome purityOvercome the cycleFinal product manufactureElectrolyte accumulators manufactureSaturated aqueous solutionElectrolyte
The invention discloses a preparation method of an NASICON-type lithium ion solid electrolyte. The method comprises the following steps: dissolving lithium nitrate and aluminum nitrate in a citric acid solution, and stirring to form a transparent and uniform nitrate mixed solution; dissolving tetrabutyl titanate in anhydrous ethanol, and stirring to form an alcoholic solution of tetrabutyl titanate; slowly adding the alcoholic solution of tetrabutyl titanate to the nitrate mixed solution, and stirring to a transparent mixed solution; dissolving ammonium biphosphate in water to obtain a saturated aqueous solution of ammonium biphosphate, adding the saturated aqueous solution of ammonium biphosphate to the transparent mixed solution in a dropwise manner, and stirring to obtain an emulsion; adjusting the pH value of the emulsion, and drying the emulsion to obtain an xerogel; carrying out heat treatment of the xerogel, and grinding the obtained xerogel to form fine powder which is precursor powder; and compacting the precursor powder to form a green body, and sintering to obtain NASICON-type lithium ion solid electrolyte slices. The method has the advantages of low energy consumption, simplicity, easy implementation, and convenience for large-scale industrialized production, and the obtained solid electrolyte has a high conductivity.
Owner:HUAZHONG UNIV OF SCI & TECH
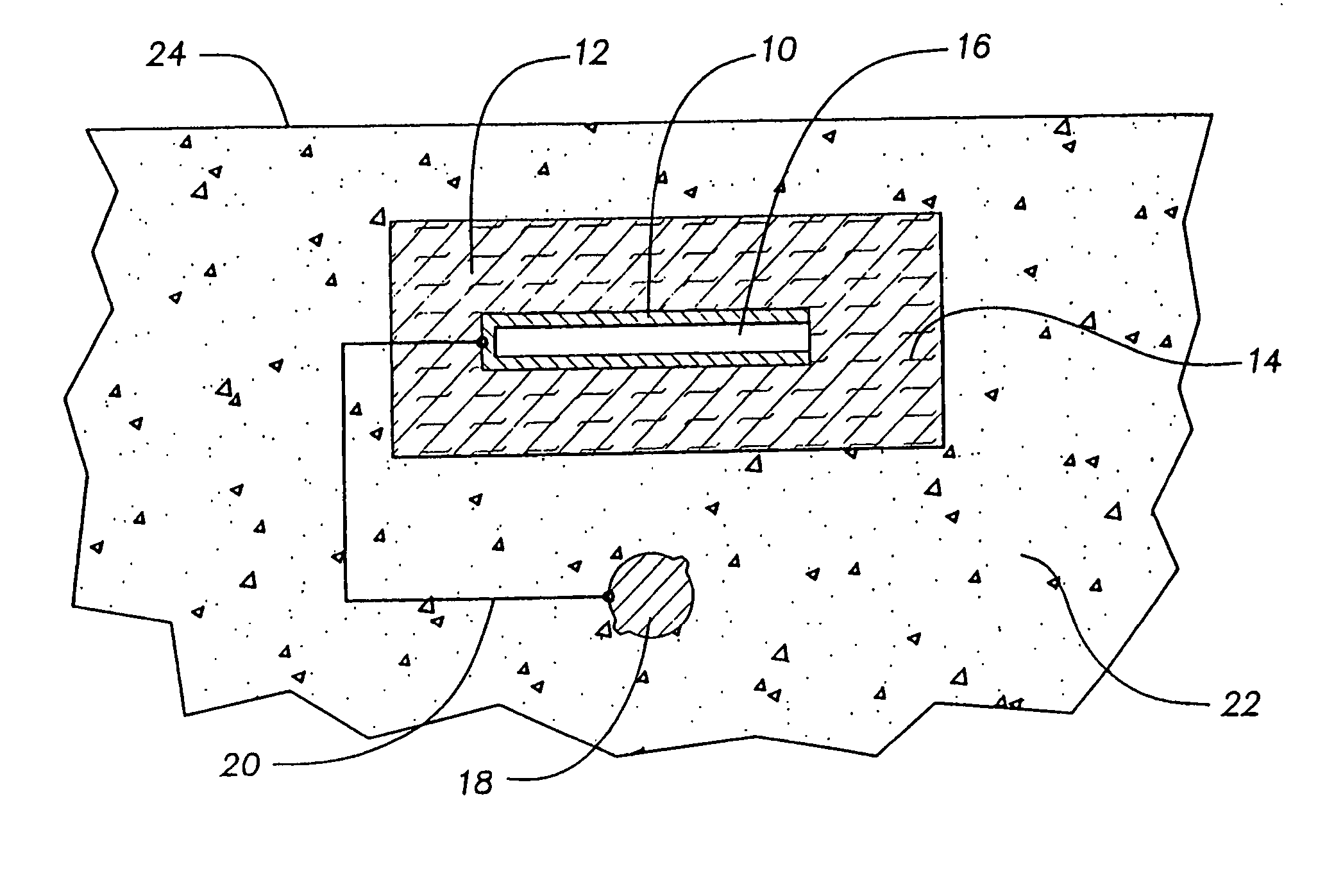
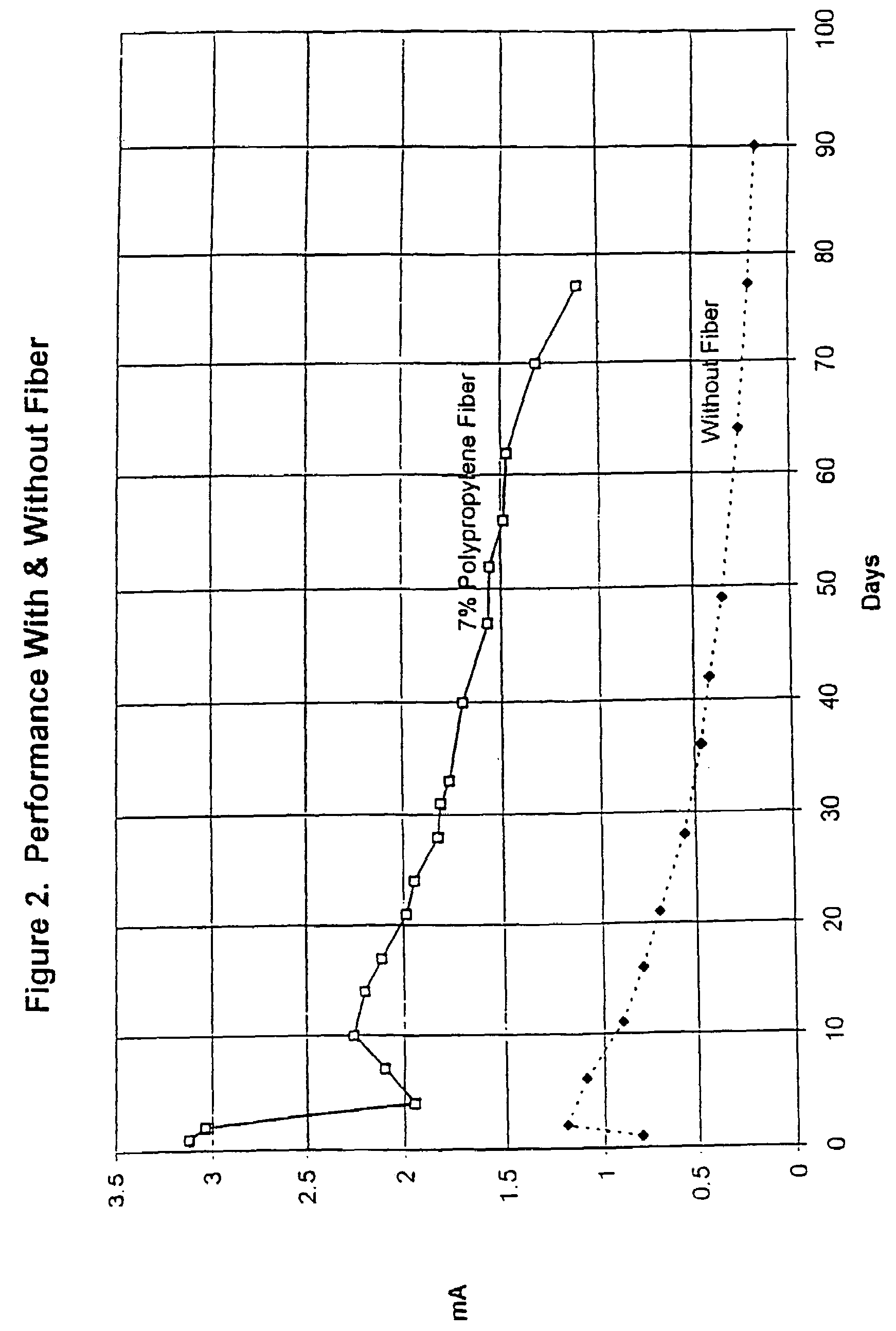
Cathodic protection system
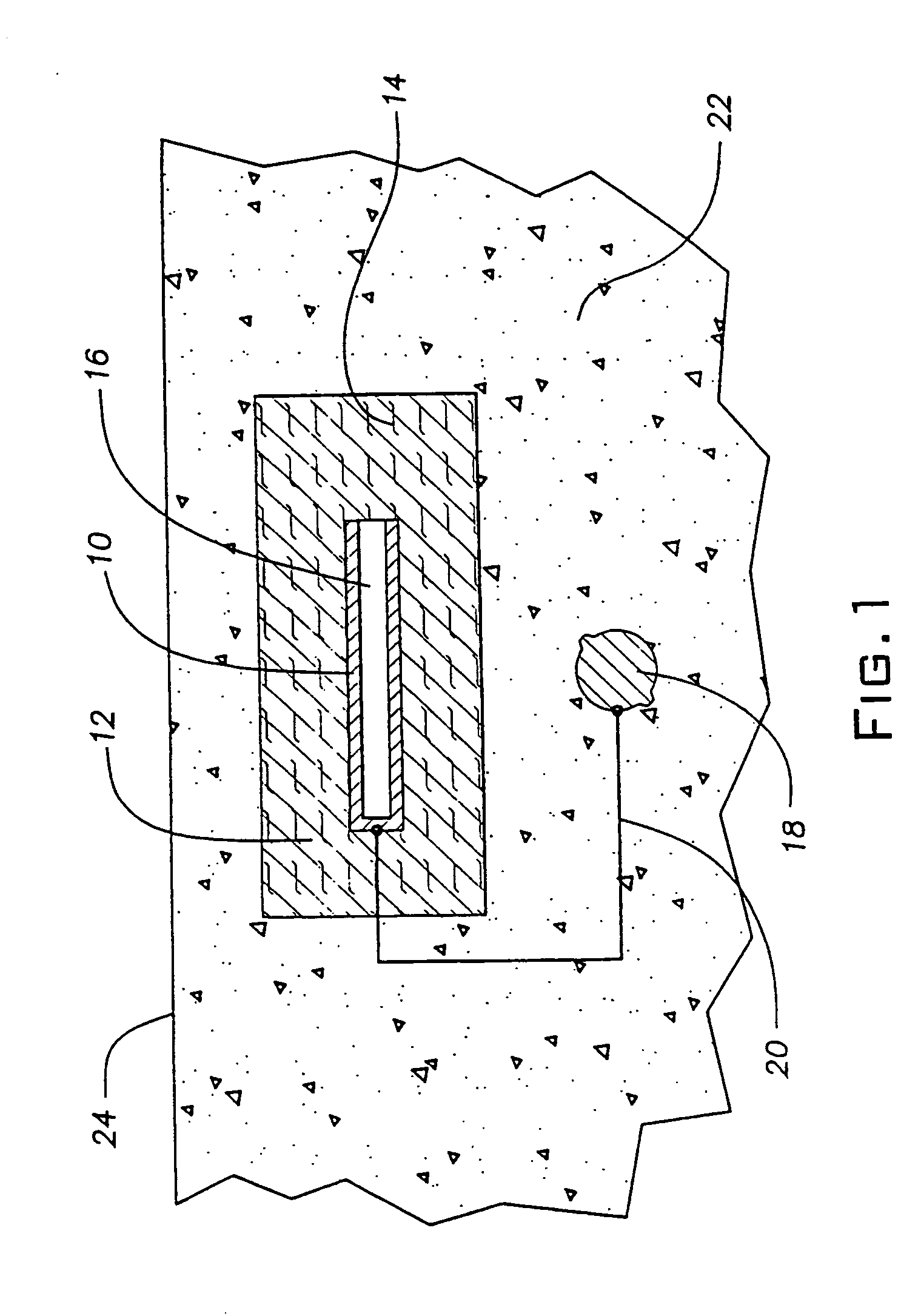
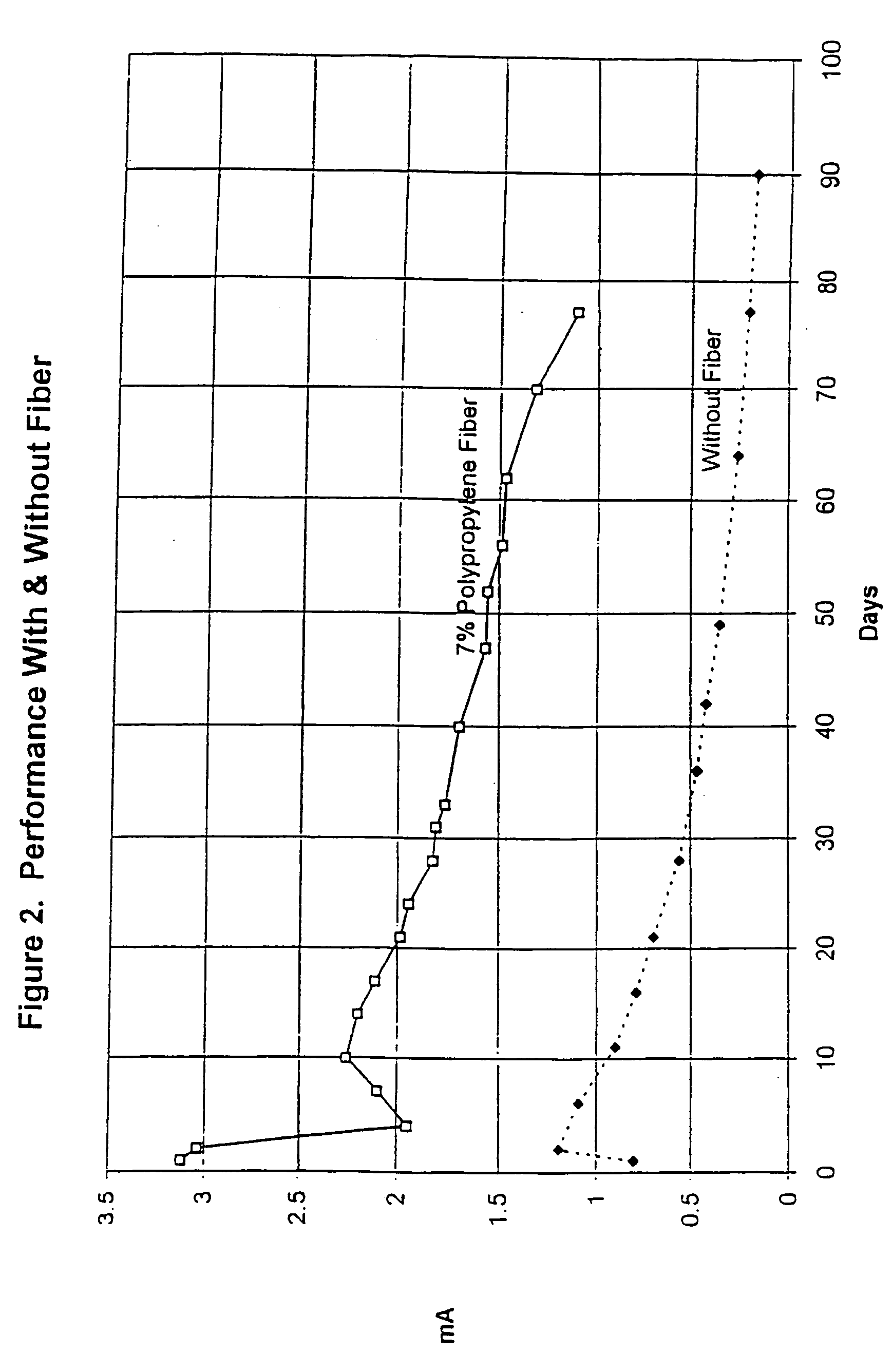
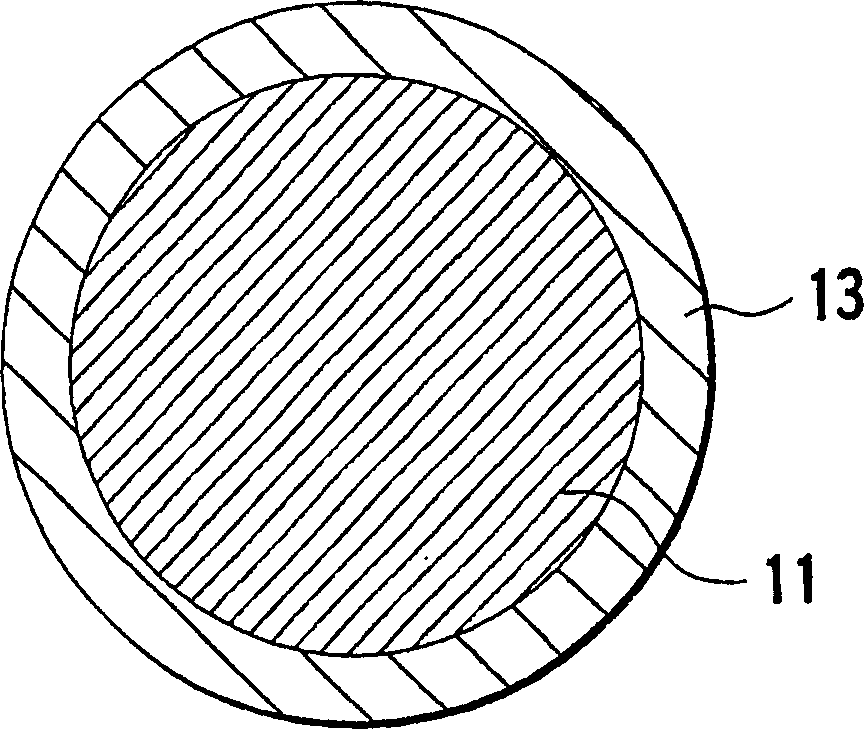
The cathodic protection system of a concrete structure (22) uses sacrificial anodes such as zinc, aluminum and alloys thereof embedded in mortar. A humectant is employed to impart high ionic conductivity to the mortar in which the anode is encapsulated. Lithium nitrate and lithium bromide and combinations thereof are preferred as the humectant. The anode (10) is surrounded by a compressive conductive matrix (12) incorporating a void volume between 15% and 50% to accommodate the sacrificial corrosion products of the anode. A void space of at least 5% of the total volume of the anode (12) may be provided opposite to the active face of the anode. Synthetic fibers such as polypropylene, polyethylene, cellulose, nylon and fiberglass have been found to be useful for forming the matrix. A tie wire is used to electrically connect the anode to the reinforcing bar.
Owner:THE EUCLID CHEM CO
Manufacture method of lithium lanthanum titanium oxide
ActiveCN1970455AImprove electrical performanceThe experiment process is simpleCell electrodesLithium compoundsAlcoholLithium-ion battery
The invention discloses a making method of Li-La-Ti oxide in the lithium ion battery domain, which comprises the following steps: adopting lithium nitrate, lanthanum nitrate and butyl titanate as raw material with molecular formula as Li3xLa2 / 3-xTiO3 (0<x<0.16); setting the molar rate of Li, La and Ti at 1: 1: 2; adopting alcohol or ethylene glycol monomethyl ether as solvent; or adjusting pH value under 1 through water and acetate; drying solution; sintering under 800-900 deg.c for 2h; obtaining pure LLTO. the invention shortens experimental period and synthesizing temperature with grain size about 200nm, which possesses excellent compact and electric property.
Owner:TSINGHUA UNIV
Positive pole material of lithium ion battery with nanometer structure and preparation method thereof
The invention discloses a positive pole material of a lithium ion battery with a nanometer structure and a preparation method thereof. The positive pole material is in a particle type core-shell structure, a core material consists of nanometer lithium iron phosphate, lithium vanadium phosphate or cobalt lithium oxide and graphene, and a shell material is porous carbon. The preparation method comprises the following steps of: taking lithium acetate, lithium oxalate, ammonium dihydrogen phosphate, ammonia metavanadate, phosphoric acid, lithium nitrate, cobalt nitrate and graphite oxide as raw materials, adopting a sol-gel method or a ball milling method to prepare a mixture, presintering the mixture in vacuum to obtain the core material, and mixing, grinding and calcining the core material with an organic carbon source to obtain the positive pole material with the particle type core-shell structure. The positive pole material has the advantages of good conductivity, good circulating performance, high capacity, small and uniform particle size, and simple preparation process and is easy for industrialized production.
Owner:深圳清研紫光科技有限公司
Doped spherical LiMn2O4 (manganese acid lithium) and preparation method thereof
The invention relates to doped spherical LiMn2O4 (manganese acid lithium) and a preparation method thereof, belonging to the technical field of new energy materials. The preparation method comprises the following steps: reaction is carried out on water-soluble manganese salt and permanganate or persulfate under the liquid-phase condition, and spherical MnO2 (manganese dioxide) is first generated by controlling the pH value, temperature and the feeding speed of reaction liquid; the spherical MnO2 is uniformly mixed with LiOH (lithium hydroxide) or LiNO3 (lithium nitrate) or Li2CO3 (lithium carbonate) and a doping agent (zinc, aluminum or chromium) so as to obtain a mixture; the mixture is calcined under the temperature of 500 to 850 DEG C and is naturally cooled along with a furnace; and then the doped spherical LiMn2O4 is obtained through ball milling. The preparation process has the characteristics of simple preparation method, low energy consumption, easy material obtaining and high efficiency; an obtained product has excellent physical, chemical and electrochemical performances. Therefore, the doped spherical LiMn2O4 is the excellent anode material for the lithium ion battery.
Owner:孙琦
Modified diaphragm special for Li-S battery, preparation method of modified diaphragm and Li-S battery
ActiveCN105280867AIncrease capacityPromote circulationCell seperators/membranes/diaphragms/spacersSecondary cellsCalcinationDimethyl ether
In a modified diaphragm special for a Li-S battery, the surface at one side, close to a positive electrode, of a general diaphragm is coated with a Ketjen black coated metal oxide modified coating layer added with a conductive agent. A preparation method of the modified diaphragm special for the Li-S battery comprises the following steps of sequentially mixing Ketjen black and an aqueous solution of a metal oxide inorganic salt, carrying out dispersion, drying and calcinations to prepare a paste, and applying the paste at one side, close to the positive electrode, of a commercial diaphragm to obtain the modified diaphragm. The invention also discloses the Li-S battery applying the modified diaphragm special for the Li-S battery. The Li-S battery is prepared by taking metal lithium as a negative electrode, applying a Ketjen balck-sulfur composite material onto an aluminum foil to serve as the positive electrode, taking a mixture of LiTFSI, lithium nitrate, 1,3-dioxolane and glycol dimethyl ether as an electrolyte and assembling the modified diaphragm special for the Li-S battery. With the modified diaphragm disclosed by the invention, a "shuttle effect" of polysulfide lithium in the Li-S battery can be prevented, the electrochemical performance and the capacity of the Li-S battery are improved, the cycle lifetime of the Li-S battery is prolonged, and the modified diaphragm is suitably used for production at a large scale.
Owner:CHANGSHA RES INST OF MINING & METALLURGY
Preparation method of composite cathode material of lithium iron phosphate and carbon nano-tubes
InactiveCN101710615AStrengthens the conductive networkIncrease contactCell electrodesWater bathsParticulates
The invention relates to a preparation method of a composite cathode material of lithium iron phosphate and carbon nano-tubes. The preparation method comprises the following steps of: (1) preparing a precursor by taking citric acid, lithium nitrate, ferric nitrate, and ammonium dihydrogen phosphate as reactants; (2) adding the carbon nano-tubes to the precursor, wherein the mass percentage of the addition the carbon nano-tubes is 1-5 percent relative to the precursor, and thoroughly mixing the carbon nano-tubes with the precursor; (3) heating the mixed liquor of the carbon nano-tubes and the precursor, which treated in the step (2), in a constant temperature water bath with the temperature of 80-90 DEG C till solvent volatilize completely, pre-sintering the dried precursor at 250-350 DEG C for 8-10 hours under nitrogen atmosphere, and preserving the temperature at 700-800 DEG C for 8-10 hours to obtain the composite cathode material of the lithium iron phosphate and the carbon nano-tubes. Since the carbon nano-tubes are added into the sol-gel precursor, on the basis of active particulate carbon cladding provided by a sol-gel method, electric conduction network of carbon is enhanced though using the carbon nano-tubes to improve contacts among particles, thereby improving electrical property of a lithium ion battery.
Owner:辽宁凯信工业技术工程有限公司
Carbon-coated doping modified lithium titanate and preparation method thereof
ActiveCN102969492ASolve the problem that the granularity is difficult to controlImprove conductivityCell electrodesHigh rateMixed materials
The invention discloses carbon-coated doping modified lithium titanate and a preparation method thereof. The carbon-coated doping modified lithium titanate is characterized in that carbon-coated doping modified lithium titanate has a general formula of Li[4-x]MgxTi[5-y]AlyO12 / C, wherein x is not less than 0.05 and not greater than 0.5, and y is not less than 0.02 and not greater than 0.25. The preparation method sequentially comprises the following steps of: dispersing titanium dioxide, soluble sugar and aluminium powder or iron powder in absolute ethyl alcohol, stirring to obtain a suspension, drying to obtain a paste, performing heat treatment to obtain carbon-coated titanium dioxide, soaking with hydrochloric acid or sulphuric acid, and preparing carbon-coated titanium dioxide with micro pores on a coating layer via washing, drying and grinding; weighing a lithium source, carbon-coated titanium dioxide, a magnesium source and an aluminium source, and mixing; calcining the mixed materials, and preserving heat; and grinding and sieving to obtain the carbon-coated doping modified lithium titanate. The carbon-coated doping modified lithium titanate and the preparation method thereof disclosed by the invention have the greatest advantage that the conductivity of the material is remarkably improved and a high discharge specific capacity and an excellent cycling stability are shown under a high rate. The method is simple in process, low in raw material price and available in raw materials, and easily realizes industrialized production. The carbon-coated doping modified lithium titanate and the preparation method thereof have a wide application prospect in the fields of negative materials for small, power and energy-storing lithium ion batteries.
Owner:HUI ZHOU BTR NEW MATERIAL TECH
Production process for composite oxide, positive-electrode active material for lithium-ion secondary battery and lithium-ion secondary battery
A composite oxide is produced via the following:a raw-material mixture preparation step of preparing a raw-material mixture by mixing a metallic-compound raw material and a molten-salt raw material with each other,the metallic-compound raw material at least including one or more kinds of Mn-containing metallic compounds being selected from the group consisting of oxides, hydroxides and metallic salts that include one or more kinds of metallic elements in which Mn is essential,the molten-salt raw material including lithium hydroxide and lithium nitrate, and exhibiting a proportion of the lithium hydroxide with respect to the lithium nitrate (i.e., (Lithium Hydroxide) / (Lithium Nitrate)) that falls in a range of from 0.05 or more to less than 1 by molar ratio;a molten reaction step of reacting said raw-material mixture at from 300° C. or more to 550° C. or less by melting it: anda recovery step of recovering said composite oxide being generated from said raw-material mixture that has undergone the reaction.
Owner:TOYOTA IND CORP
Preparation method of NASICON type lithium fast ion conductor
InactiveCN103466588AImprove ionic conductivitySecondary cellsPhosphorus compoundsElectrical conductorDiammonium phosphate
The invention discloses a preparation method of an NASICON type lithium fast ion conductor. The preparation method comprises the following steps: adding butyl titanate into a citric acid solution, uniformly stirring the solution, adding a citric acid solution of lithium nitrate, aluminum nitrate and diammonium phosphate, uniformly stirring the solution and adding ethylene glycol, heating the solution to a certain temperature, and stirring the solution to completely gelatinize the same; drying the gel to obtain dry gel, and grinding and calcining the dry gel to obtain precursor powder; and grinding the precursor powder fine powder, carrying out isostatic press molding on the fine powder on a tablet press to obtain an electrolyte sheet of the NASICON type lithium fast ion conductor. The preparation method is used for reducing the sintering temperature of the material, perfecting the sintering performance of the material and improving the density of the material to improve the ionic conductivity of the material. The ionic conductivity of the NASICON type lithium fast ion conductor prepared by the preparation method reaches 6.34*10<-4> S / cm (25 DEG C), so that compared with a traditional preparation method, the ionic conductivity is significantly improved.
Owner:HUAZHONG UNIV OF SCI & TECH
Positive electrode material for non-aqueous electrolyte lithium ion battery and battery using the same
PROBLEM TO BE SOLVED: To provide a positive electrode material for a nonaqueous electrolyte lithium ion battery, which can suppress a decomposition of an electrolyte even when moisture does not enter a cell and a battery is charged or discharged at high temperature.SOLUTION: The positive electrode material for a nonaqueous electrolyte lithium ion battery includes lithium-nickel oxide having a surface covered with an Li compound to be attached. The Li compound is at least one selected from the group consisting of lithium phosphate, an LiPON compound, an Li<SB POS="POST">2< / SB>O-B<SB POS="POST">2< / SB>O<SB POS="POST">3< / SB>compound, an Li<SB POS="POST">2< / SB>O-B<SB POS="POST">2< / SB>O<SB POS="POST">3< / SB>-LiI compound, an Li<SB POS="POST">2< / SB>S-SiS<SB POS="POST">2< / SB>compound, an Li<SB POS="POST">2< / SB>S-SiS<SB POS="POST">2< / SB>-Li<SB POS="POST">3< / SB>PO<SB POS="POST">4< / SB>compound, lithium hydroxide, lithium fluoride, lithium acetate, lithium acetylide ethylenediamine, lithium benzoate, lithium bromide, lithium carbonate, lithium nitrate, lithium oxalate, lithium pyruvate, lithium stearate, lithium tartrate and lithium sulfate.
Owner:ENVISION AESC JAPAN LTD
Method for preparing Nano tube of titanate
InactiveCN101003385ALarge specific surface areaImproved electrical and catalytic propertiesPolycrystalline material growthTitanium compoundsCopper nitrateSodium titanate
This invention discloses a method for preparing titanate nanotubes. The method comprises: uniformly mixing nitrate and power of sodium titanate nanotubes at a mol ratio of (5-100):1, performing melt-reaction at 50-450 deg.C for 3-48 h, cooling, washing to remove unreacted nitrate, and drying to obtain titanate nanotubes. The nitrate is lithium nitrate, and drying to obtain titanate nanotubes. The nitrate is lithium nitrate, potassium nitrate, rubidium nitrate, cesium nitrate, silver nitrate, nickel nitrate, thallium nitrate, copper nitrate, zinc nitrate or cobalt nitrate. The precursor of sodium titanate nanotubes is prepared by: reacting titanium dioxide, metatitanic acid or titanate ester in 20-80 wt. % NaOH aqueous solution at 100-140 deg.C for 12-72 h, washing with water, filtering and drying. This invention can prepare titanate nanotubes with large specific surface area by melt-exchange method.
Owner:HENAN UNIVERSITY
Cathodic protection system
Owner:THE EUCLID CHEM CO
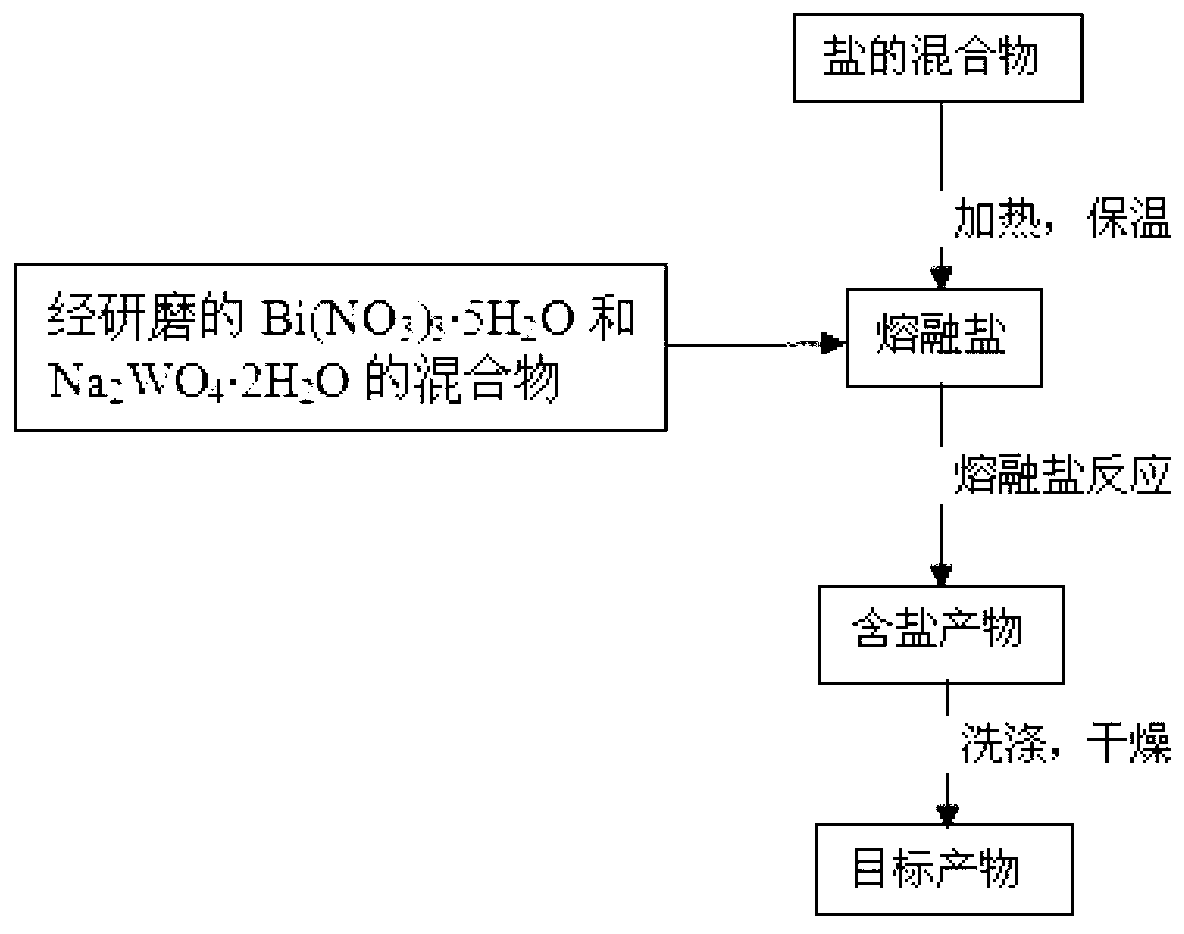
Nanometer photocatalyst bismuth tungstate and preparation method thereof
InactiveCN103157461APressure no requirementSimple ingredientsMetal/metal-oxides/metal-hydroxide catalystsTungstateSodium nitrate
The invention discloses a method for preparing nanometer bismuth tungstate by utilizing a fused salt method. According to the method, bismuth nitrate and sodium tungstate are adopted as raw materials, potassium nitrate / sodium nitrate and lithium nitrate are utilized as a fuse salt system, the raw materials are mixed and added into a molten salt system in a crucible, and the nanoscale bismuth tungstate powder is obtained through reaction. According to the method, the uniform mixing of raw material on a molecule scale can be realized, and the nanoscale bismuth tungstate powder with high purity is obtained. The method provided by the invention has the advantages that the equipment is simple, the technological process is short, the operation is simple, the product performance is excellent, and the method has an extensive application prospect.
Owner:NANJING UNIV OF SCI & TECH
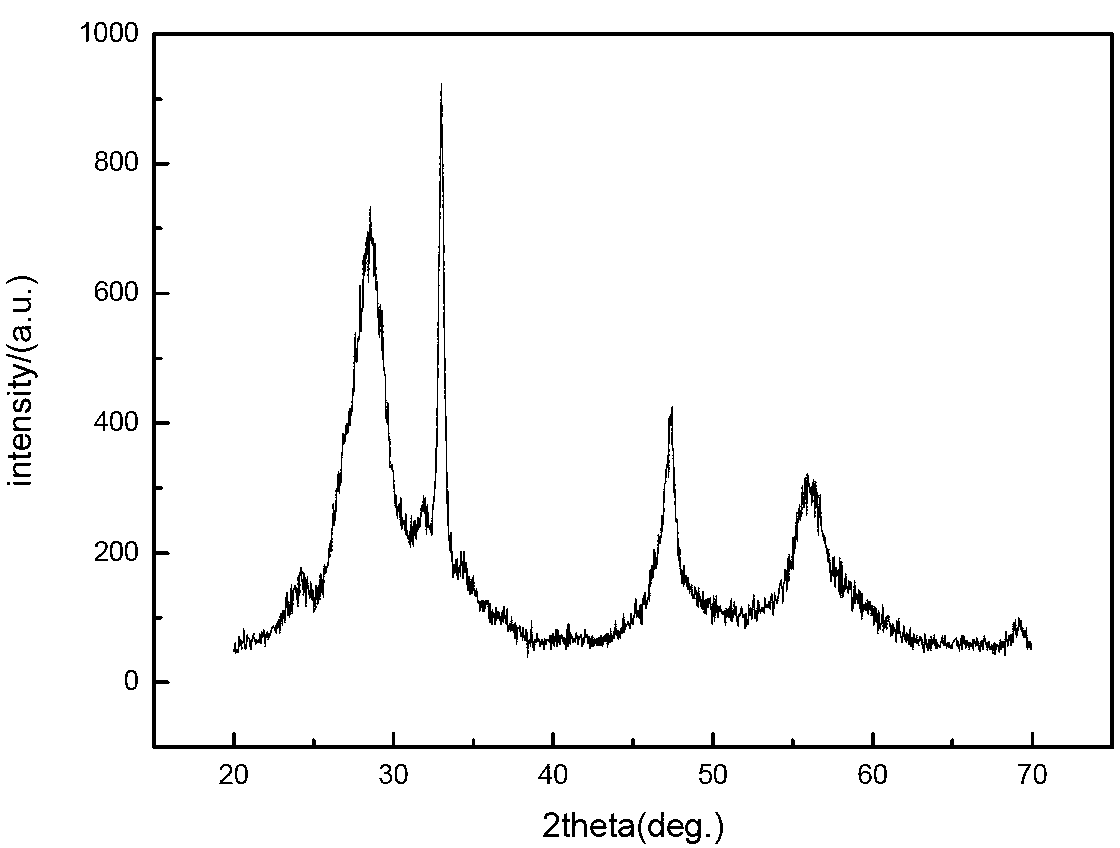
Carbuncle type yellow fluorescent material Y3Al5O12:Ce,Li preparation method
The preparation method for garnet yellow-light fluorescent material Y3Al5O12:Ce,Li comprises following steps: selecting material with mole proportion: (3-x-y) yttrium nitrate, x cerous nitrate, y lithium nitrate and 5 aluminum nitrate, wherein, 0.01<=x<=0.1, 0.01<=y<=0.08; determining x and y for proper nitrate to dissolve into deionized water; mixing evenly, adding citrate complexing agent with mole number as 1.6-3.2 times to the aluminum nitrate; heating and evaporizing the solution in water bath at 60-90Deg; adding nitric acid to control solution pH value between 4 and 7; mixing continually to form gel; combusting the gel slowly at 200-280Deg to obtain the precursor powder; calcining the powder at 800-1200Deg to obtain the product.
Owner:SHANGHAI INST OF OPTICS & FINE MECHANICS CHINESE ACAD OF SCI
Lithium-air battery for electric vehicles and other applications using molten nitrate electrolytes
InactiveUS20160028133A1Minimization requirementsReduce oxygenFuel and secondary cellsHybrid cell detailsElectrical batteryLithium–air battery
Owner:MILES MELVIN H
Production process for composite oxide, positive-electrode active material for lithium-ion secondary battery and lithium-ion secondary battery
A composite oxide is produced via the following: a raw-material mixture preparation step of preparing a raw-material mixture by mixing a metallic-compound raw material and a molten-salt raw material with each other, the metallic-compound raw material at least including one or more kinds of Mn-containing metallic compounds being selected from the group consisting of oxides, hydroxides and metallic salts that include one or more kinds of metallic elements in which Mn is essential, the molten-salt raw material including lithium hydroxide and lithium nitrate, and exhibiting a proportion of the lithium hydroxide with respect to the lithium nitrate (i.e., (Lithium Hydroxide) / (Lithium Nitrate)) that falls in a range of from 0.05 or more to less than 1 by molar ratio; a molten reaction step of reacting said raw-material mixture at from 300° C. or more to 550° C. or less by melting it: and a recovery step of recovering said composite oxide being generated from said raw-material mixture that has undergone the reaction.
Owner:TOYOTA IND CORP
Visible light response method for preparing Bi2WO6 photocatalyst fused salt
InactiveCN101264934ALow costSimple processBismuth compoundsMetal/metal-oxides/metal-hydroxide catalystsLight responseSodium nitrate
The invention discloses a preparation method of fused salt of a visible light response Bi2WO6 photocatalyst. The method comprises the steps of (1) mixing lithium nitrate and sodium nitrate at a weight ratio of 27:23 as a reaction medium; (2) mixing the reaction medium and an oxide raw material at a weight ratio of (5-30):1, adding anhydrous alcohol, mixing, and oven-drying at 50-80 DEG C; (3) charging into an aluminum oxide crucible, placing a resistance furnace, heating to 200-500 DEG C at a speed of 2-5 DEG C / min, and keeping the temperature for 2-8 hours to obtain a solid reaction product; and (3) dissolving in hot water, filtering, washing, removing free compounds, and drying at 50-80 DEG C to obtain visible light response Bi2WO6 photocatalyst. The inventive method has the advantages of low synthesis temperature, short period, no need of any templates and surfactants as auxiliary materials, simple steps and equipment, low cost, easily controlled process conditions, and adaptability to industrial production.
Owner:ZHEJIANG UNIV
Preparation method of lithium silicate porous material used for absorption of high temperature CO2
ActiveCN104003411AAvoid ball millingAvoid calcinationProductsReagentsPore distributionCo2 absorption
The invention relates to a preparation method of a lithium silicate porous material used for absorption of high temperature CO2; according to the preparation method, lithium nitrate is used as a lithium source and an oxidizing agent, ethyl orthosilicate is used as a silica source, citric acid is used as a complexing agent and a fuel, a mixture of ethanol and water is used as a solvent, and a sol-gel combustion synthesis method is used. The preparation method is as follows: first, the citric acid and the lithium nitrate are dissolved in the solvent, then the ethyl orthosilicate is added to obtain a precursor by hydrolysis, sol-gelatinization, ageing and drying, after compression moulding, the precursor is ignited in air, and after combustion, the lithium silicate porous material used for absorption of high temperature CO2 can be obtained. The lithium silicate porous material used for absorption of high temperature CO2 is uniform in pore distribution and high in activity of carbon dioxide absorption. Compared with the prior art, the lithium silicate porous material is directly prepared by the sol-gel combustion synthesis method, the technological process is simplified without the need for complex and expensive equipment, and the lithium silicate porous material is easy to realize the industrialized production.
Owner:UNIV OF SCI & TECH BEIJING
One-step method for preparing high-magnification copper-doped lithium manganese dioxide lithium ion battery positive electrode material
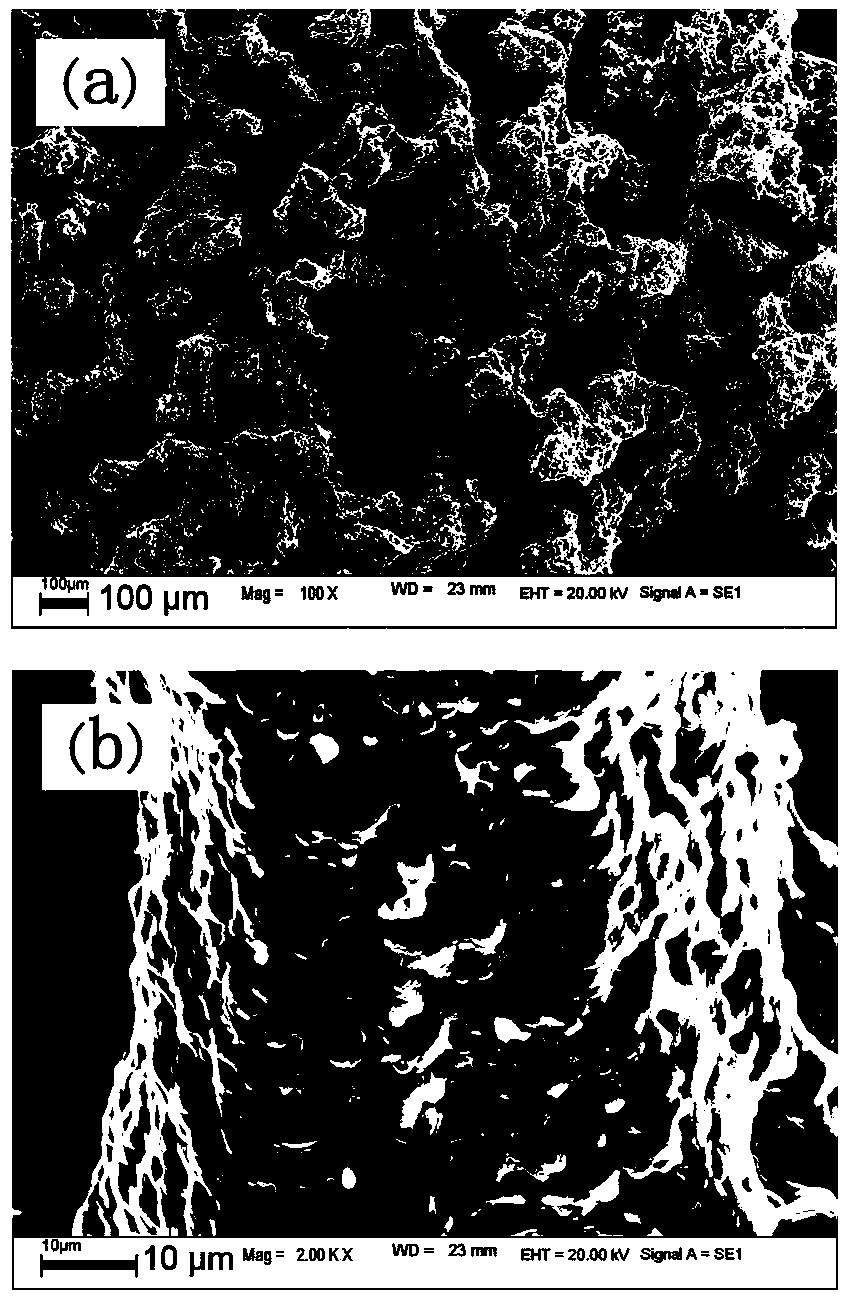
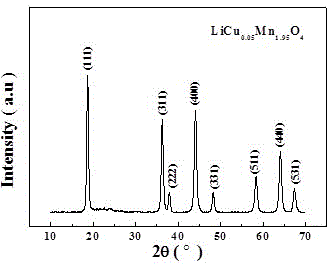
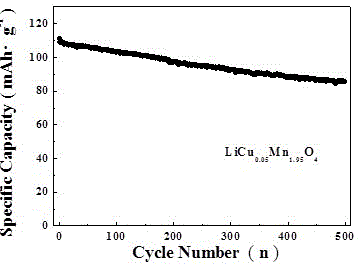
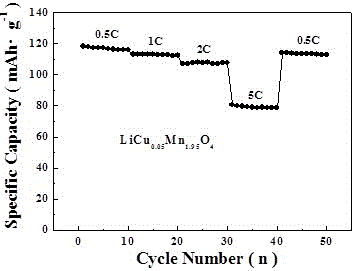
The invention discloses a preparation method of lithium manganese dioxide lithium ion battery positive electrode material with high magnification and excellent cyclic performance. The method particularly comprises the following steps of putting the reactants of lithium nitrate, manganese acetate and copper acetate into a crucible, adding a proper amount of nitric acid, combusting in a muffle furnace, and preserving heat, thereby obtaining the spinel LiCu0.05Mn1.95O2 electrode material. The preparation method of the LiCu0.05Mn1.95O4 lithium ion battery positive electrode material, which is disclosed by the invention, has the characteristics of operation simplicity, rapid synthesis speed, low cost and scale production is easy to achieve.
Owner:YUNNAN MINZU UNIV
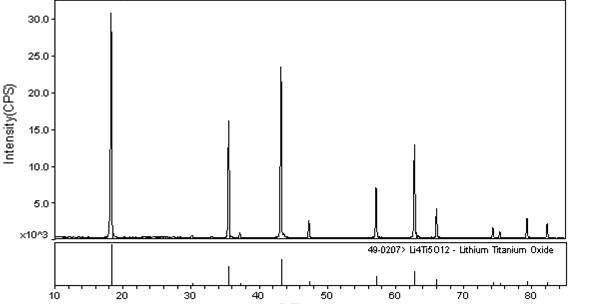
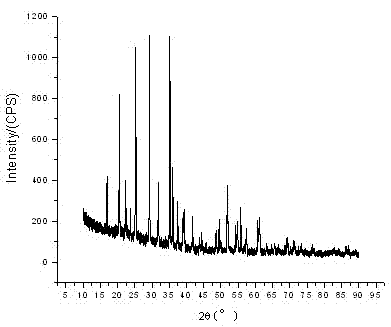
Method for preparing lithium titanate powder
InactiveCN102249297ASimple processReduce manufacturing costTitanium compoundsElectrochemistryMuffle furnace
The invention discloses a method for preparing lithium titanate powder. The method comprises the following steps of: weighing an excessive amount of lithium salt (lithium nitrate or lithium acetate and the like) with the concentration 0-5 percent according to the metering ratio of a chemical formula Li4Ti5O12, preparing the lithium salt into a solution, and adding an appropriate amount of coordination agent and incendiary agent (such as citric acid or EDTA (Ethylene Diamine Tetraacetic Acid) or oxalic acid ) into the Li<+> solution to obtain a uniform mixed solution A; weighing metatitanic acid (H2TiO3) according to the metering ratio of a chemical formula Li4Ti5O12, adding an appropriate amount of hydrogen peroxide (oxyful, H2O2) and an excessive amount of ammonia water (NH3*H2O), and adding a certain amount of coordination agent and incendiary agent (such as citric acid or oxalic acid or EDTA and the like) after the metatitanic acid is fully dissolved to obtain a uniform mixed solution B; mixing the prepared solution A with the solution B, heating and concentrating into jelly (or drying into powder at a temperature between 50 DEG C and 200 DEG C); and putting the powder obtained by drying (or the directly prepared jelly) into a muffle furnace for calcining at a temperature between 500 DEG C and 900 DEG C for 1-10 hours to obtain Li4Ti5O12 powder. The method has the advantages of simple technical process, low cost of raw materials, easiness for control of stoichiometric ratio and excellent electrochemical performance of a product, and is suitable for mass production; and the particle diameter of the powder is between 50 nanometers and 500 nanometers.
Owner:HUNAN UNIV OF HUMANITIES SCI & TECH
Method for preparing positive electrode material of LiFePO4 by phosphorous acid or salt thereof
InactiveCN101332987ALow costWide variety of sourcesCell electrodesPhosphorus compoundsPhosphorous acidPhosphate
The invention relates to a method for preparing lithium iron phosphate by phosphorous acid or phosphite; the preparation method is as follows: lithium salt, ferrous salt, phosphate and phosphorous acid or phosphite are blended, added with carbon-containing compound, and further added with wet milling liquid, processed by ball milling and mixing, and dried at normal atmosphere or vacuum; the dried powder is sintered to prepare lithium iron phosphate cathode material. The lithium is one of or the mixture of lithium carbonate, lithium hydroxide, lithium oxalate, lithium acetate, lithium nitrate, lithium fluoride and lithium phosphate; the ferrous salt is one of or the mixture of iron oxalate, iron acetate, iron dichloride, ferrous sulfate or ferrous carbonate; the phosphate is one of or the mixture of ammonium phosphate, diammonium phosphate of monoammonium phosphate; the molecular formula of phosphite is AH2PO3, A2HPO3, EHPO3, E(H2PO3)2 or G(H2PO3)3; the carbon-containing compound is one of or the mixture of polypropylene, polyacrylamide, polyvinyl alcohol, glucose, cane sugar or starch. The method of the invention has simple preparing process and short time consumed, and the materials have good cycling performance under the heavy current discharging condition.
Owner:FUJIAN NORMAL UNIV
Preparation method for manganese phosphate lithium nanosheet
InactiveCN102903918AGood dispersionFacilitated DiffusionCell electrodesMANGANESE ACETATEPolyethylene glycol
The invention relates to a preparation method for a manganese phosphate lithium nanosheet. According to the preparation method, glycol and water are used as a solvent, and polyethylene glycol is introduced, so that the formation of crystal nucleus and the growth of crystal are influenced, and as a result, the thermosynthesis of the solvent of the manganese phosphate lithium nanosheet can be achieved. The preparation method comprises the following steps of: dissolving ascorbic acid in the water / glycol solvent; then dissolving into phosphoric acid and manganese acetate in sequence; dropwise adding the water / glycol solution of manganese acetate to the previous solution containing phosphoric acid, lithium acetate and ascorbic acid; then introducing proper polyethylene glycol; fully mixing to obtain a precursor for water / solvent thermal reaction; transferring the precursor into a reaction kettle system to be sealed; thermally processing at 160 to 240 DEC G; and carrying out thermal reaction to the solvent to obtain the manganese phosphate lithium nanosheet. By adopting the preparation method, products are stable in quality, high in purity and high in dispersion of particles; the lithium ions can be dispersed well; the electrochemical performance of a lithium ion battery can be improved; and the preparation method is simple in technical process, easy to control, free of pollution, low in cost, and easy for mass production.
Owner:ZHEJIANG UNIV
A lithium sulfide battery electrolyte for improving safety and stability
InactiveCN109148956AImprove stabilityImprove securityLi-accumulatorsSecondary cells servicing/maintenanceOxalatePhosphorus pentasulfide
The invention provides a lithium sulfur battery electrolyte for improving safety and stability. The electrolyte is prepared from lithium salt, solvents and additives, The lithium salt is a mixture oflithium bis-trifluoromethanesulfonimide (LiTFSI), lithium bis-oxalate borate (LiBOB) and lithium difluorooxalate borate (LiODFB), the solvent is a mixture of dioxolane (DOL) and tetraethanol dimethylether (TEGDEM), and the additive is lithium nitrate and phosphorus pentasulfide. The phosphorus sulfide additive in the battery prepared by the electrolyte reacts with the negative electrode lithium metal to form a passivation layer, At that same time, the decomposition of lithium bis (trifluoromethanesulfonimide) on the negative electrode side can be inhibit by the action of lithium bis (oxalate)and lithium difluorooxalate, so that the stability and cycle performance of the battery can be improved. The lithium bis (trifluoromethanesulfonimide) can protect the negative electrode, effectivelyreduce the generation of lithium dendrite and improve the safety of the battery.
Owner:CHENDU NEW KELI CHEM SCI CO LTD
Rechargeable lithium-air and other lithium-based batteries using molten nitrates
ActiveUS8795868B1Reduce moisture contentMinimize amount of waterDeferred-action cellsCell electrodesLithium oxideMolten salt
A rechargeable molten salt electrolyte battery has an anode comprising lithium, a cathode electrode comprising a conductive metal that is compatible with the nitrate melt, an electrolyte comprising lithium nitrate or lithium nitrate mixtures with other nitrates which electrolyte is capable of becoming an ionic conductive liquid upon being heated above its melting point, wherein oxygen for reaction at the cathode or within the melt is provided from an external source to be delivered to the cathode through the electrolyte and provision is made to collect lithium oxide formed during discharge to be reconstituted as lithium ions and oxygen during recharge. At least a portion of the oxygen reduction reaction is provided by a nitrate ion pathway.
Owner:MILES MELVIN H
Method for manufacturing glass substrate for data storage medium and glass substrate
InactiveUS20110135963A1Improve stabilityHigh strengthMagnetic materials for record carriersBase layers for recording layersPotassium nitrateMolten salt
The present invention relates to a method for manufacturing a glass substrate for data storage mediums, the method including a chemical strengthening treatment step of dipping a glass for a substrate including, in terms of mol % on the basis of oxides, from 58 to 66% of SiO2, from 9 to 15% of Al2O3, from 7 to 15% of Li2O and from 2 to 9% of Na2O, provided that Li2O+Na2O is from 13 to 21%, in a mixed molten salt to form a compressive layer on front and back surfaces of the glass for a substrate, in which the mixed molten salt includes, in terms of mass percent, from 1 to 7.5% of lithium nitrate, from 28 to 55% of sodium nitrate and from 40 to 69% of potassium nitrate.
Owner:ASAHI GLASS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com