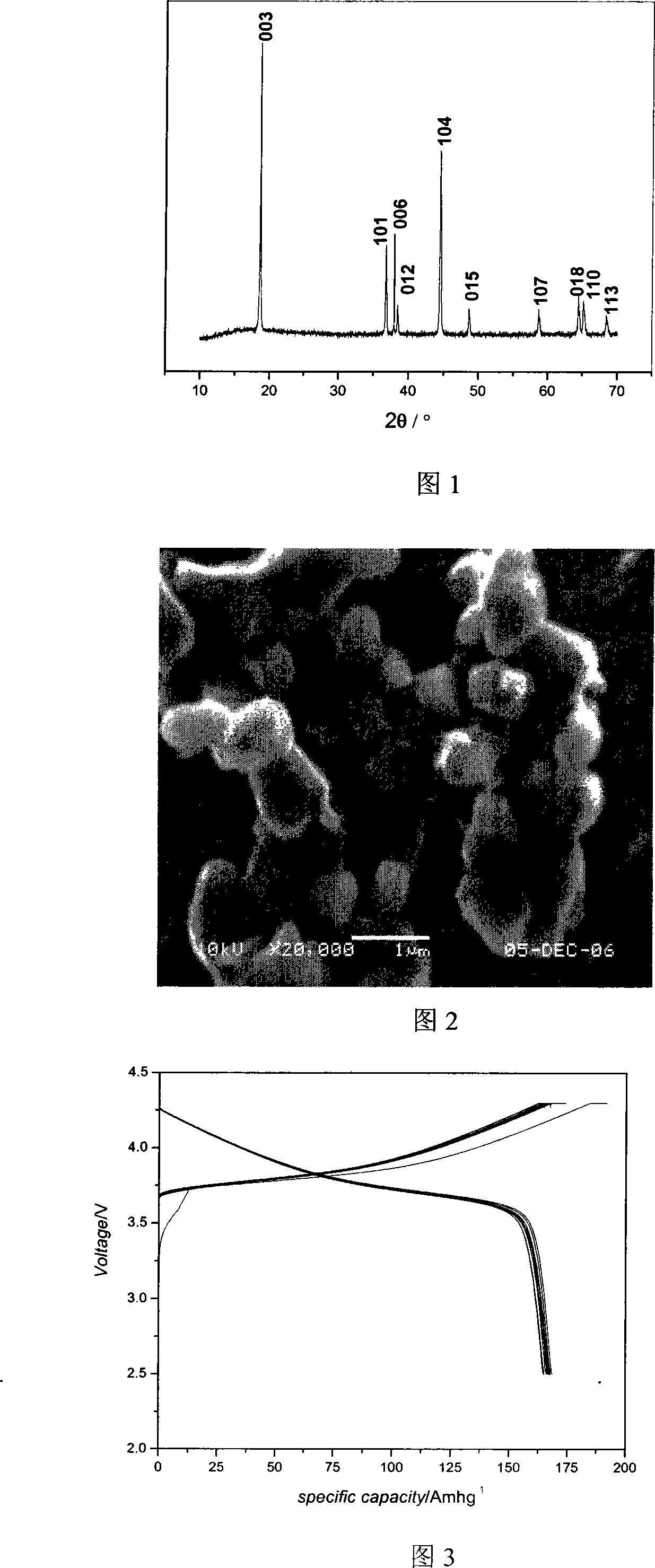
Coprecipitation-combustion synthesis method for lithium nickel cobalt manganate
A nickel-cobalt lithium manganese oxide, combustion synthesis technology, applied in the direction of nickel compounds, chemical instruments and methods, inorganic chemistry, etc., can solve the problems of irregular positive electrode material particles, high equipment requirements, large production energy consumption, etc., to overcome useful Component loss, good chemical stability, and the effect of saving washing water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0019] With Ni(CH 3 COO) 2 4H 2 O, Co(CH 3 COO) 2 4H 2 O and Mn(CH 3 COO) 2 4H 2 O, lithium nitrate is the main raw material, ammonia water is the complexing agent, with H 2 C 2 o 4 ·H 2 O is a precipitant, and LiNi is synthesized by co-precipitation-combustion method 1 / 3 co 1 / 3 mn 1 / 3 o 2 .
[0020] Steps:
[0021] 1. According to n(Ni):n(Co):n(Mn) (molar ratio) = 1:1:1, accurately weigh Ni(CH 3 COO) 2 4H 2 O, Co(CH 3 COO) 2 4H 2 O and Mn(CH 3 COO) 2 4H 2 O, adding an appropriate amount of distilled water to make a mixed solution with a concentration of 1M;
[0022] 2. Add ammonia water to the above mixed solution to make a complex solution.
[0023] 3. Mole ratio n(C 2 o 4 2- ):n(Ni+Co+Mn)=1.1:1, accurately weigh H 2 C 2 o 4 ·H 2 O, grind it into powder with a mortar and add it to the above complex solution.
[0024] 4. Stir at a speed of 600r / min in a water bath reactor at 40°C, and react for 30nm.
[0025] 5. Adjust the pH to 8.5 with amm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com