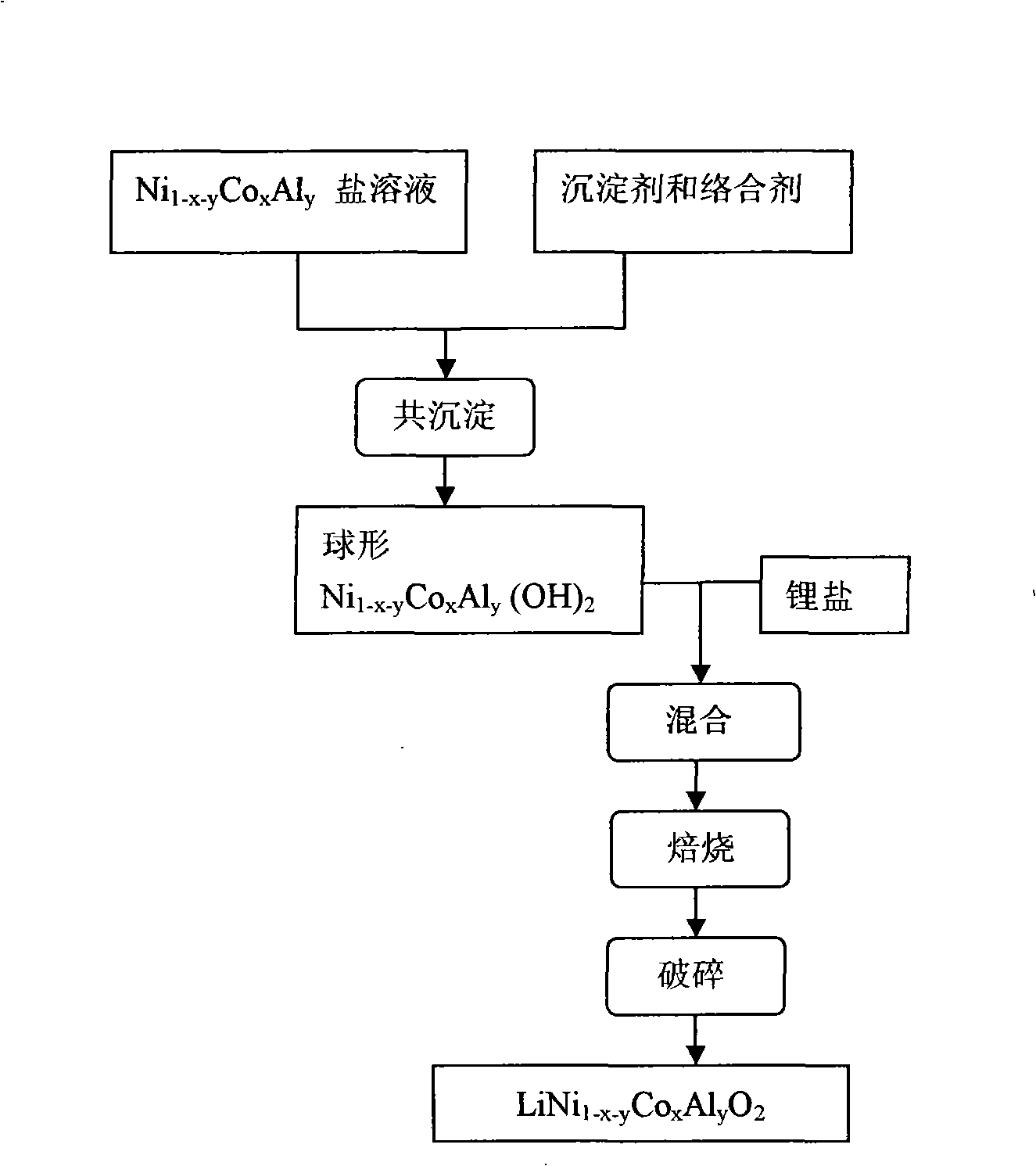
Spherical aluminum-doped nickel cobalt lithium for lithium ion battery and its making method
A technology for lithium-alnico-cobaltate and lithium-ion batteries, which is applied in the field of spherical positive-electrode materials for lithium-ion batteries doped with aluminum-nickel-cobaltate lithium and its preparation. The preparation method is cumbersome and difficult to achieve the effects of controllable powder particle size distribution and shape, improved charge-discharge cycle stability, and low product price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
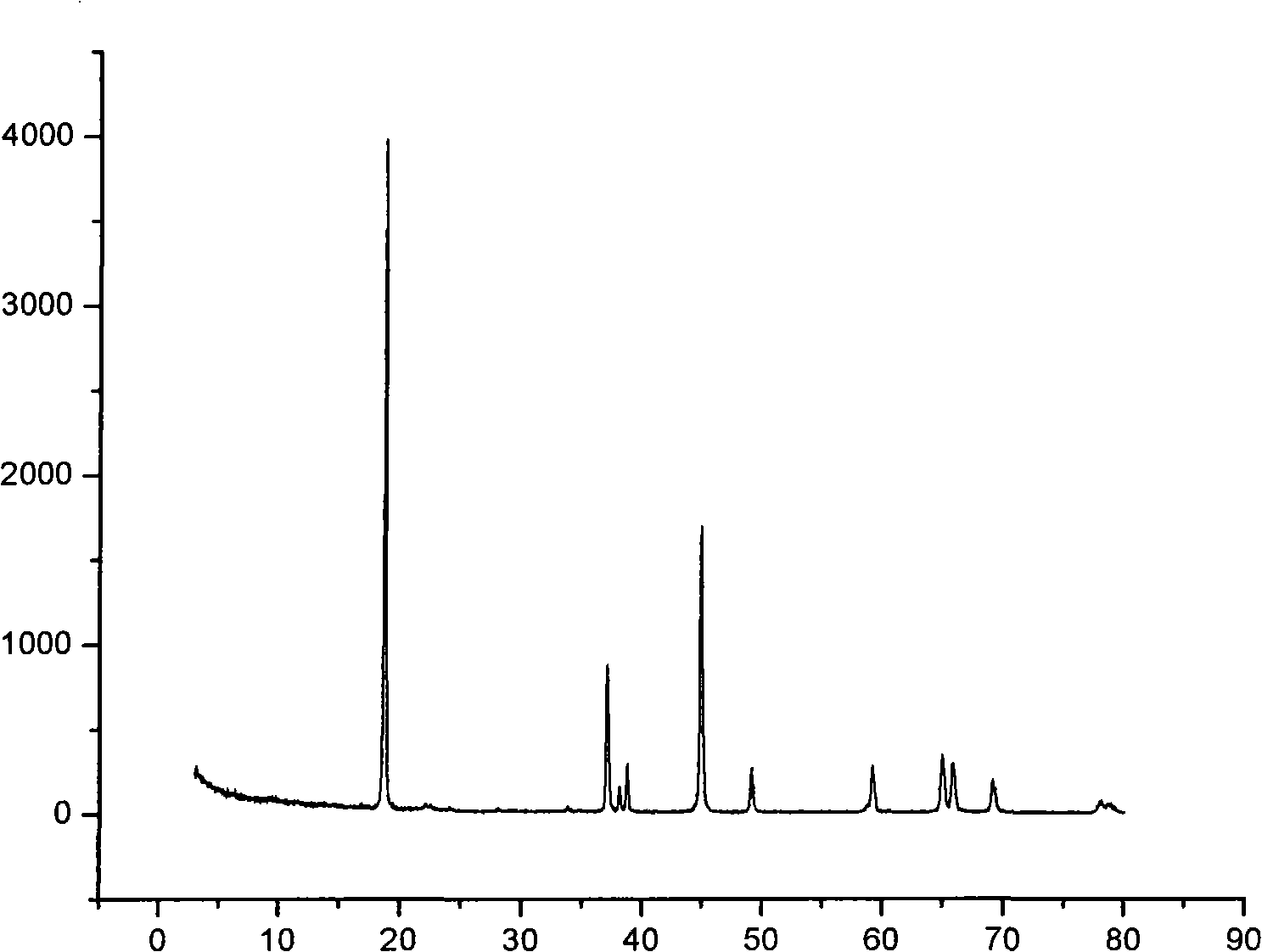
[0024] First, nickel nitrate, cobalt nitrate and aluminum nitrate were mixed in a molar ratio of 0.8:0.15:0.05 to prepare a 2M aqueous solution, sodium hydroxide was prepared to 2L of a 6M solution, and 0.4L of 1M ammonia water was added. Then the prepared salt solution is added into a 10L reaction kettle filled with 2000ml 0.5M sodium carbonate aqueous solution at a flow rate of 0.5L / h, stirred and heated to 25°C, and the feed rate of the prepared alkali solution is adjusted to control the reaction The pH value of the system is between 10.0 and 10.2. After 24 hours, the particle size of the precursor in the solid-liquid mixture reaches 12.077. After the feeding is completed, the spherical aluminum-nickel-cobalt-doped lithium precursor is separated. The electron microscope photo is as follows image 3 shown. Then the prepared precursor was washed and filtered with deionized water, and then dried at 120° C. for 24 h. Lithium hydroxide and the precursor were mixed evenly at a r...
example 2
[0027] According to the method of Example 1, the precursor of aluminum-doped nickel-cobalt hydroxide was prepared and mixed with lithium salt, and the temperature was controlled at 750° C. for 24 hours. After cooling, it was crushed and classified to obtain a spherical aluminum-nickel-cobaltate lithium material.
[0028] After inspection, the aluminum-nickel-cobalt oxide has a tap density of 2.12g / cm3, a particle size of 9.421μm, an initial capacity of 181mAh / g, a 100-cycle capacity retention rate of 84%, and an initial charge-discharge efficiency of 91%.
example 3
[0030] According to the method of Example 1, the precursor of aluminum-doped nickel-cobalt hydroxide was prepared and mixed with lithium salt, and the temperature was controlled at 800° C. for 24 hours. After cooling, it was crushed and classified to obtain a spherical aluminum-nickel-cobaltate lithium material.
[0031] After inspection, the aluminum-nickel-cobalt oxide has a tap density of 2.21g / cm3, a particle size of 10.215μm, an initial capacity of 171mAh / g, a 100-cycle capacity retention rate of 83%, and an initial charge-discharge efficiency of 84%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tap density | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com