Method for preparing lithium titanate powder
A technology of lithium titanate and powder, which is applied in the field of preparation of lithium titanate powder, can solve the problems of difficult removal and high energy consumption, and achieve the effects of simple process, excellent electrochemical performance and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
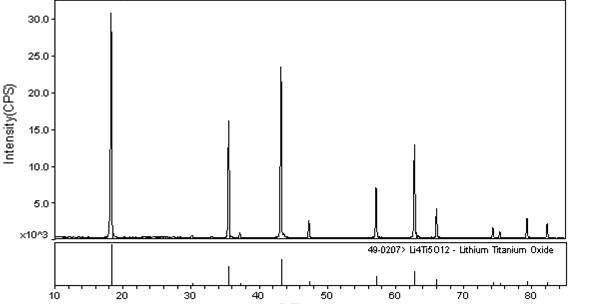
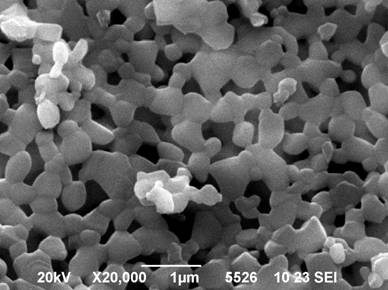
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation method of lithium titanate powder described in this embodiment comprises the following steps:
[0029] 1), according to the chemical formula Li 4 Ti 5 o 12 Metering ratio Weigh an excess of 0-5% lithium acetate, make a solution, and then add to Li + Add appropriate amount of complexing agent and burning agent EDTA to the solution to prepare uniform mixed solution A.
[0030] 2), according to the chemical formula Li 4 Ti 5 o 12 Metering ratio Weigh metatitanic acid (H 2 TiO 3 ), and then add an appropriate amount of hydrogen peroxide (hydrogen peroxide, H 2 o 2 ) and excess ammonia (NH 3 ×H 2 O), after the metatitanic acid is completely dissolved, add a certain amount of citric acid to obtain a uniform mixed solution B.
[0031] 3) Mix the A and B solutions prepared above, then concentrate the mixed solution into a jelly, dry it at 100°C to make a powder, or make a powder by spray drying.
[0032] 4) Put the powder (or directly ...
Embodiment 2
[0034] 1), according to the chemical formula Li 4 Ti 5 o 12 Metering ratio Weigh an excess of 0-5% lithium nitrate to make solution A.
[0035] 2), according to the chemical formula Li 4 Ti 5 o 12 Metering ratio Weigh metatitanic acid (H 2 TiO 3 ), and then add an appropriate amount of hydrogen peroxide (hydrogen peroxide, H 2 o 2 ) and excess ammonia (NH 3 ×H 2 O), after the metatitanic acid is completely dissolved, add a certain amount of oxalic acid to obtain a uniform mixed solution B.
[0036] 3) Mix the A and B solutions prepared above, then concentrate the mixed solution to a jelly, and dry it at 120°C to make a powder, or make a powder by spray drying.
[0037] 4) Put the above-prepared powder (or the directly-prepared jelly) into a muffle furnace and calcinate at 800°C for 4 hours to obtain Li 4 Ti 5 o 12 Powder.
Embodiment 3
[0039] 1), according to the chemical formula Li 4 Ti 5 o 12 Metering ratio Weigh an excess of 0-5% lithium acetate, make a solution, and then add to Li + Add appropriate amount of complexing agent and burning agent EDTA to the solution to prepare uniform mixed solution A.
[0040] 2), according to the chemical formula Li 4 Ti 5 o 12 Metering ratio Weigh metatitanic acid (H 2 TiO 3 ), and then add an appropriate amount of hydrogen peroxide (hydrogen peroxide, H 2 o 2 ) and excess ammonia (NH 3 ×H 2 O), after the metatitanic acid is completely dissolved, add a certain amount of citric acid to obtain a uniform mixed solution B.
[0041] 3) Mix the A and B solutions prepared above, then concentrate the mixed solution to a jelly, dry it at 100°C to make a powder, or make a powder by spray drying.
[0042] 4) Put the above-prepared powder (or the directly-prepared jelly) into a muffle furnace and calcinate at 800°C for 6 hours to obtain Li 4 Ti 5 o 12 Powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com