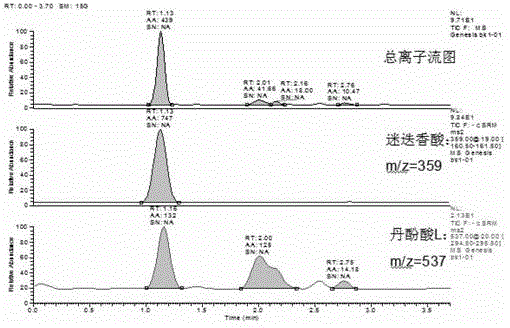
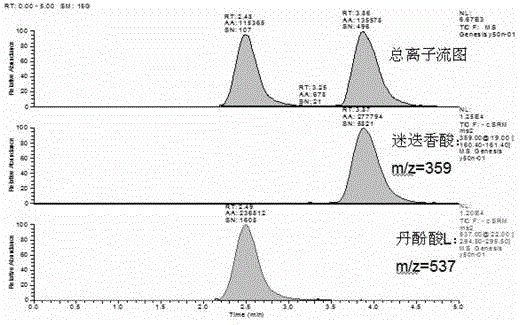
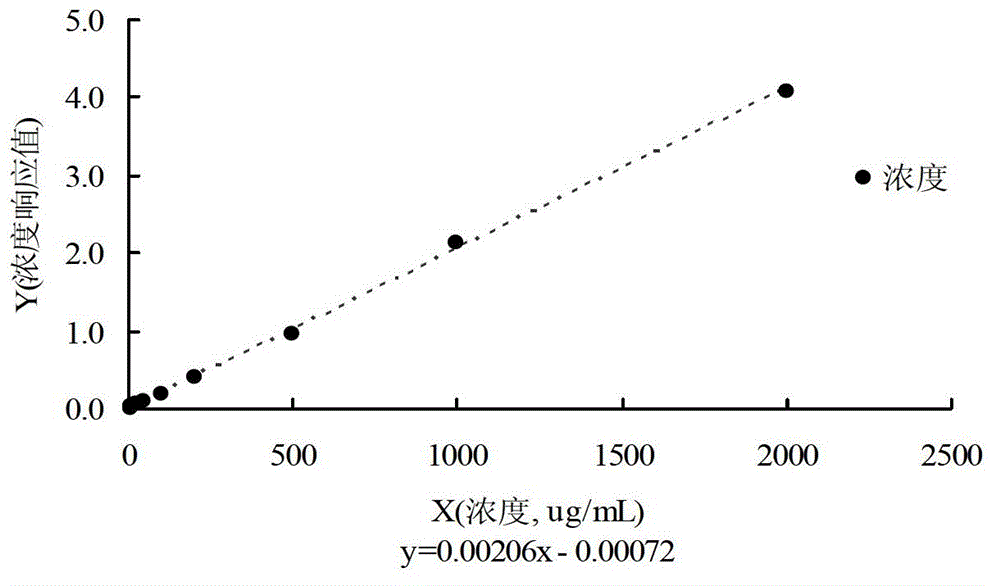
Method for determining salvianolic acid L in blood plasma
A technology of salvianolic acid and blood plasma, applied in the field of drug content in plasma, can solve the problems of low sensitivity, low content, complex structure of salvianolic acid L, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Plasma sample pretreatment:
[0100] 200uL plasma + 50uL 1M hydrochloric acid + 20uL internal standard (rosmarinic acid), vortexed for 30s, added 3mL ethyl acetate, vortexed for 4min, centrifuged to take the ethyl acetate layer, and dried in a 30°C water bath with nitrogen. After drying, reconstitute with 100uL mobile phase (0.1% formic acid water: acetonitrile = 7:3), vortex for 3min, centrifuge at 12000r / min for 3min, take the supernatant and inject 20uL.
[0101] Determination conditions:
[0102] Chromatographic column: Agilent ZORBAX Eclipse XDB-C18Analytical 2.1×150mm3.5-Micron Mobile phase: A: 0.1% formic acid water, B: acetonitrile.
[0103] Elution conditions: 0-3.7min, A:B=70:30 (v / v) isocratic elution, flow rate 0.25mL / min.
[0104] Mass spectrometry conditions: negative ion scanning, spray voltage -3000V, shell gas 5arb, auxiliary gas 20arb, capillary temperature 350°C. SRM detection mode is used to detect ion pairs: salvianolic acid L m / z537-295, collisi...
Embodiment 2
[0106] Plasma sample pretreatment:
[0107] 200uL plasma + 50uL 0.01M hydrochloric acid + 20uL internal standard (rosmarinic acid), vortexed for 10s, added 1mL ethyl acetate, vortexed for 1min, centrifuged to take the ethyl acetate layer, and dried in a water bath with nitrogen at 10°C. After drying, reconstitute with 100uL mobile phase, vortex for 3min, centrifuge at 12000r / min for 1min, take supernatant and inject 20uL.
[0108] Determination conditions:
[0109] Chromatographic column: Agilent ZORBAX Eclipse XDB-C18Analytical 2.1×150mm3.5-Micron Mobile phase: A: 0.02% formic acid water, B: acetonitrile.
[0110] Elution conditions: 0-3.7min, A:B=30:70 (v / v) isocratic elution, flow rate 0.1mL / min.
[0111] Mass spectrometry conditions: negative ion scanning, spray voltage -2500V, shell gas 1arb, auxiliary gas 5arb, capillary temperature 250°C. SRM detection mode is used to detect ion pairs: salvianolic acid L m / z537-493, collision energy (CE)=5eV, rosmarinic acid m / z359-1...
Embodiment 3
[0113] Plasma sample pretreatment:
[0114] 200uL plasma + 50uL 5M sulfuric acid + 20uL internal standard (danshensu), vortexed for 3min, added 3mL of petroleum ether, vortexed for 10min, centrifuged to take the petroleum ether layer, and dried with nitrogen in a water bath at 60°C. After drying, reconstitute with 100uL mobile phase, vortex for 3min, centrifuge at 15000r / min for 10min, take supernatant and inject 20uL.
[0115] Determination conditions:
[0116] Chromatographic column: Agilent ZORBAX Eclipse XDB-C8Analytical2.1×150mm3.5-Micron Mobile phase: A: 5% formic acid water, B: acetonitrile.
[0117] Elution conditions: 0-3.7min, A:B=90:10 (v / v) isocratic elution, flow rate 0.4mL / min.
[0118] Mass spectrometry conditions: negative ion scanning, spray voltage -3500V, shell gas 15arb, auxiliary gas 30arb, capillary temperature 400°C. SRM detection mode was used to detect ion pairs: salvianolic acid L m / z537-185, collision energy (CE)=40eV, danshensu m / z197-135, CE=30e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com