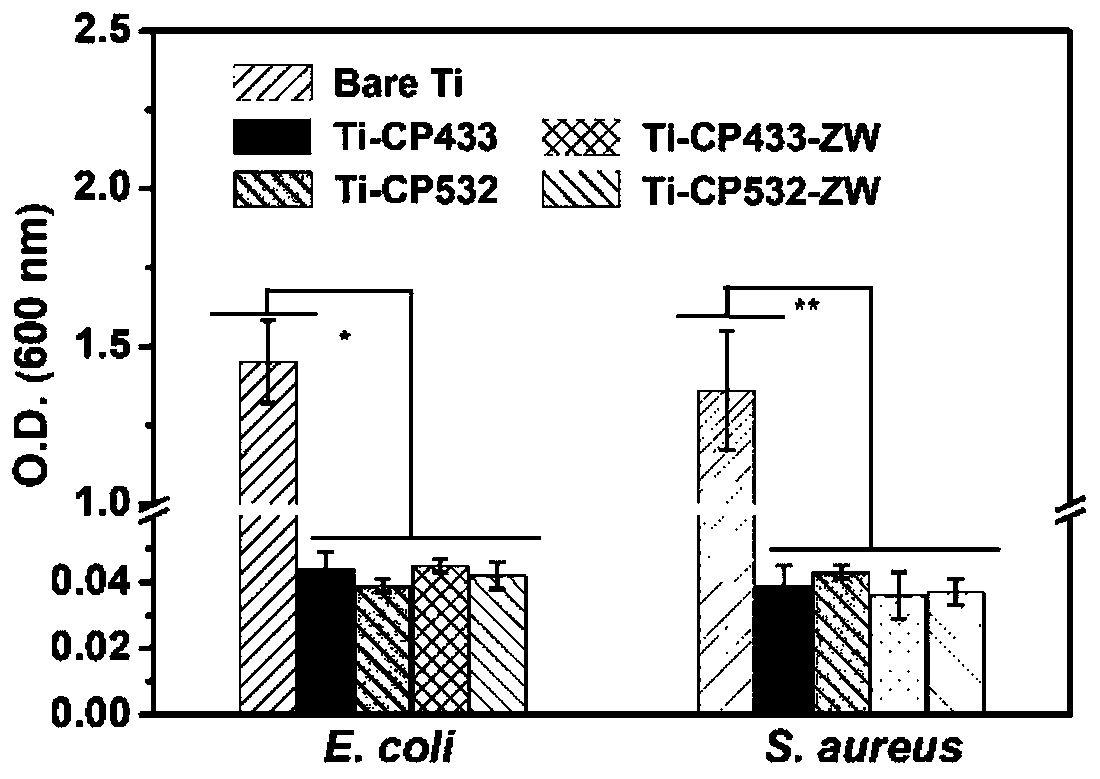
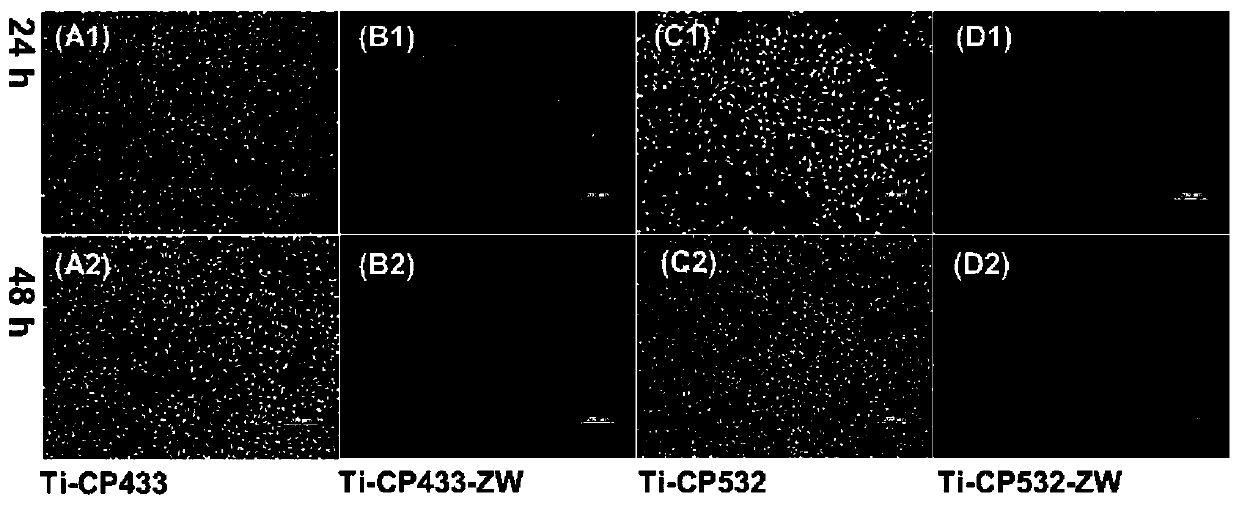
Carboxylic acid betaine zwitterionic composite antibacterial functional coating material and preparation method and application thereof
A technology of carboxybetaine and zwitterions, applied in coatings, antifouling/underwater coatings, biocide-containing paints, etc., can solve lengthy, lack of biocompatibility, superhydrophilic polymer coating fixation And the problems such as cumbersome preparation method, to achieve the effect of enhanced hardness and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
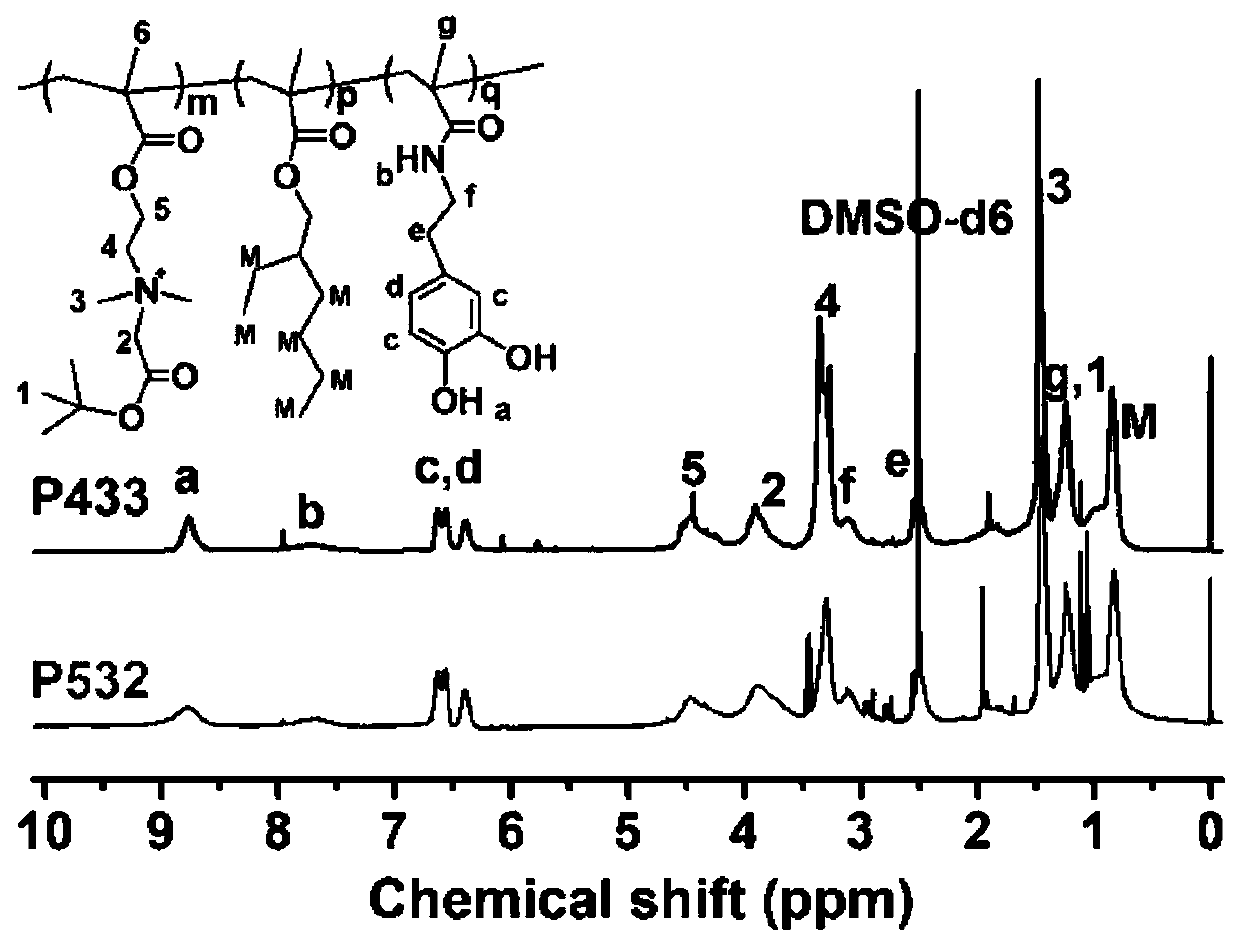
[0039] According to the preparation process of step S1, using DMF as a solvent, a certain amount of tert-butyl protected carboxybetaine (CB-tBu), isooctyl acrylate (EHA), dopamine methacrylamide (DOPA-Aa) according to The molar ratio n[CB-tBu]:n[EHA]:n[DOPA-Aa] was 5:3:2, put it into a round bottom flask, and the concentration of the reaction solution was 1.0g / mL; 1.5% added initiator AIBN, pass N 2 After deoxygenation, evacuate and seal, place in a 68°C oil bath with magnetic stirring for 24 hours, after the reaction is completed, cool to room temperature, use anhydrous ether as a precipitant, repeat purification 3 times, and finally vacuum dry to constant weight to obtain Polymer P(CB-tBu-co-EHA-co-DOPA-Aa), abbreviated as P532, was stored in a dry box. Polymer P532 1 HNMR spectrum as figure 1 shown.
[0040] Then prepare a 20 mg / mL ethanol solution of polymer P532, and add isobutanol dropwise to the above polymer ethanol solution at a rate of 20 μL / min under stirring cond...
Embodiment 2
[0047] According to the preparation process of step S1, using DMF as a solvent, a certain amount of tert-butyl protected carboxybetaine (CB-tBu), isooctyl acrylate (EHA), dopamine methacrylamide (DOPA-Aa) according to The molar ratio n[CB-tBu]:n[EHA]:n[DOPA-Aa] is 4:3:3 and put into the round bottom flask, the concentration of the reaction solution is 1.0g / mL; % added initiator AIBN, thru N 2 After deoxygenation, evacuate and seal, place in a 68°C oil bath with magnetic stirring for 24 hours, after the reaction is completed, cool to room temperature, use anhydrous ether as a precipitant, repeat purification 3 times, and finally vacuum dry to constant weight to obtain Polymer P(CB-tBu-co-EHA-co-DOPA-Aa), abbreviated as P433, was stored in a dry box. Polymer P433 1 H NMR spectrum as figure 1 shown.
[0048] Prepare a 20mg / mL polymer P433 ethanol solution, add isobutanol dropwise to the above polymer ethanol solution at a rate of 20 μL / min under stirring conditions, with the ...
Embodiment 3
[0058] According to the preparation process of step S1, using DMF as a solvent, a certain amount of tert-butyl protected carboxybetaine (CB-tBu), isooctyl acrylate (EHA), dopamine methacrylamide (DOPA-Aa) according to The molar ratio n[CB-tBu]:n[EHA]:n[DOPA-Aa] is 5:3:2 and put into the round bottom flask, the concentration of the reaction solution is 1.5g / mL; % added initiator AIBN, thru N 2 After deoxygenation, evacuate and seal, place in a 68°C oil bath with magnetic stirring for 24 hours, after the reaction is completed, cool to room temperature, use anhydrous ether as a precipitant, repeat purification 3 times, and finally vacuum dry to constant weight to obtain Polymer P(CB-tBu-co-EHA-co-DOPA-Aa), abbreviated as P532, was stored in a dry box.
[0059] Prepare a 10 mg / mL polymer ethanol solution, and add AgNO at a concentration of 20 μg / mL to the above polymer ethanol solution with the same volume at a rate of 20 μL / min under stirring conditions. 3 Ethanol solution, aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydrodynamic diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com