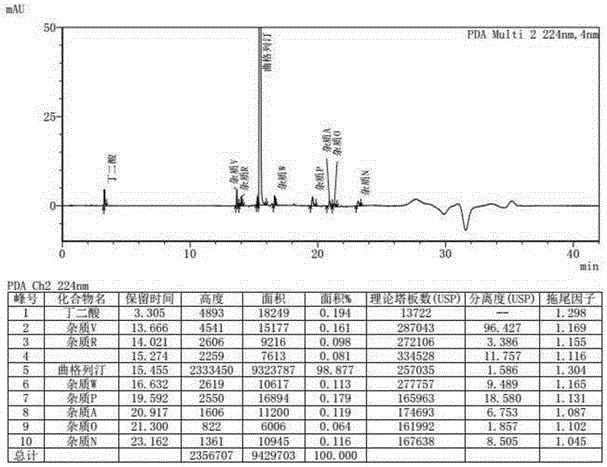
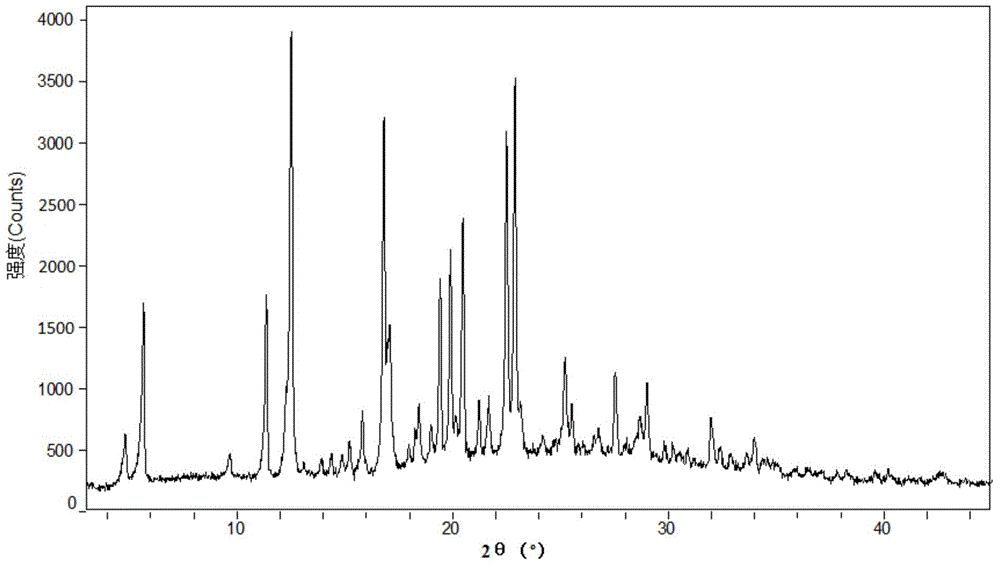
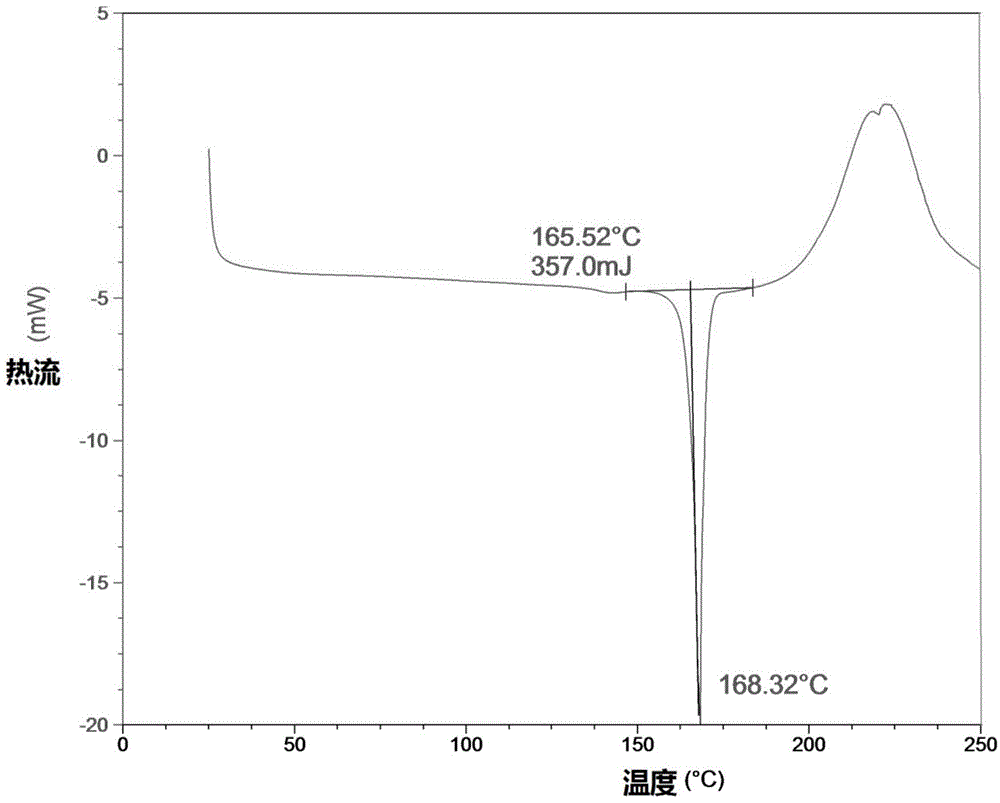
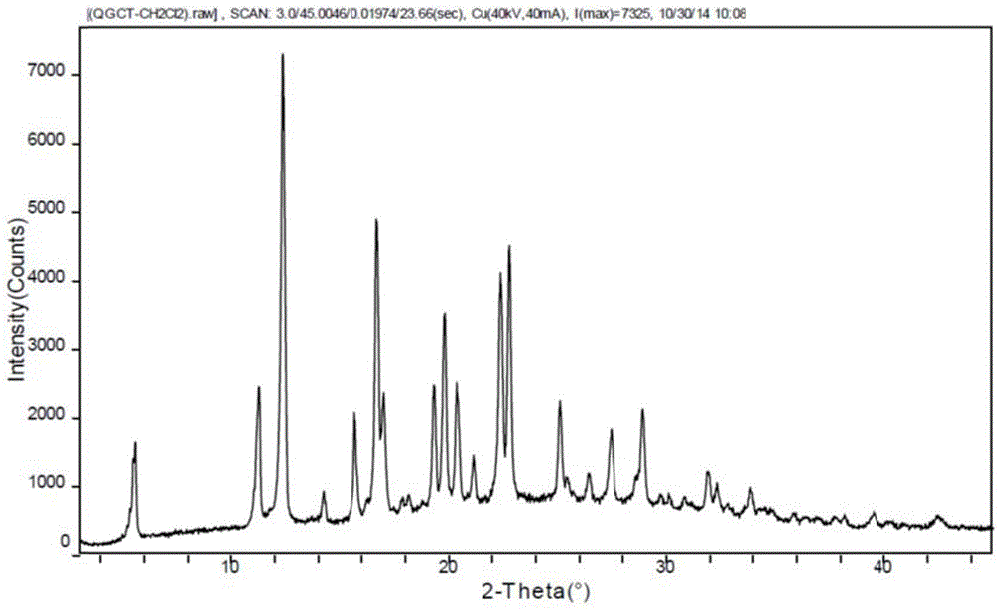
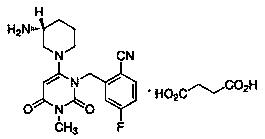
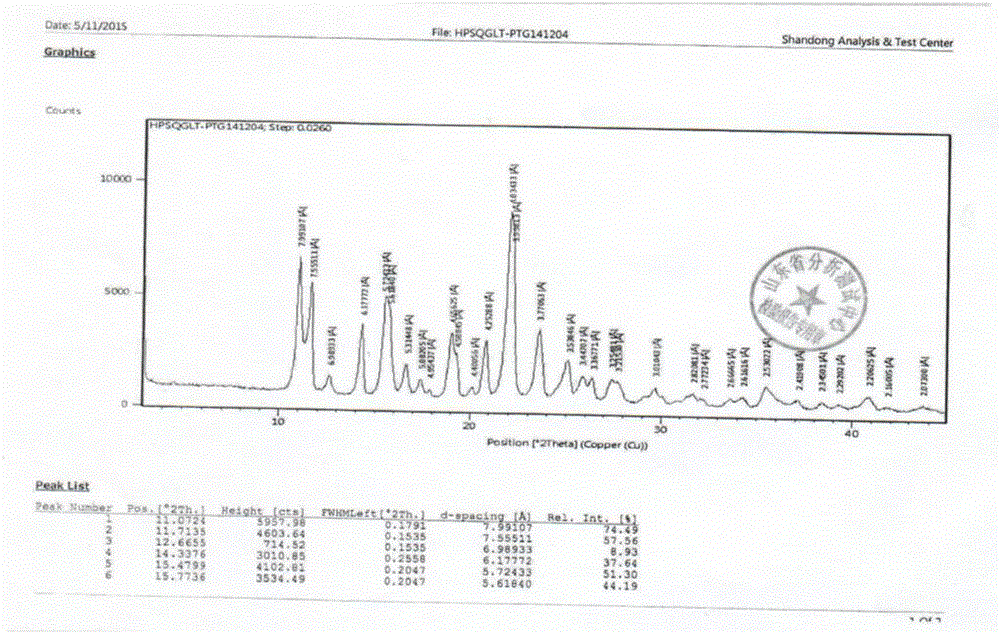
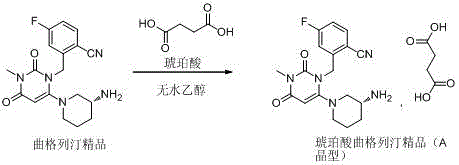
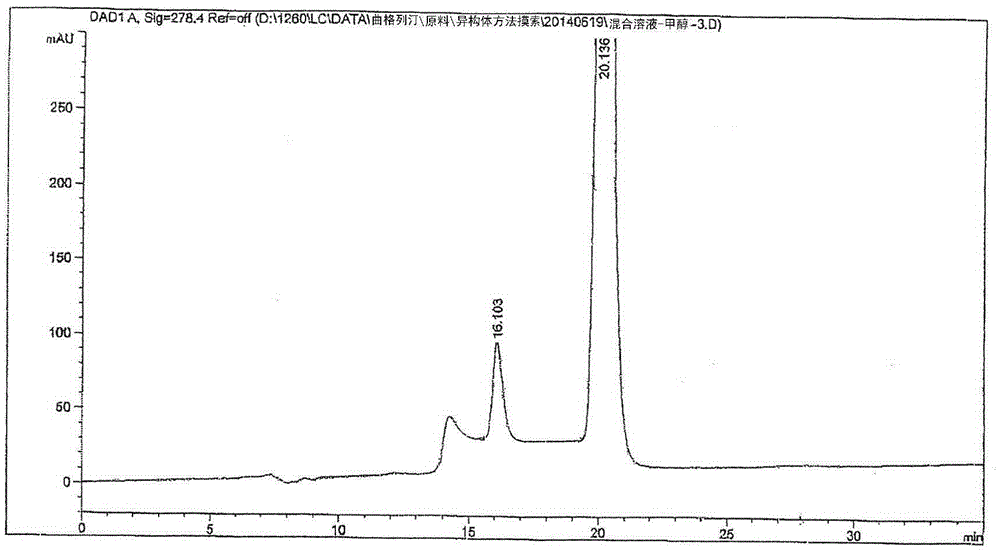
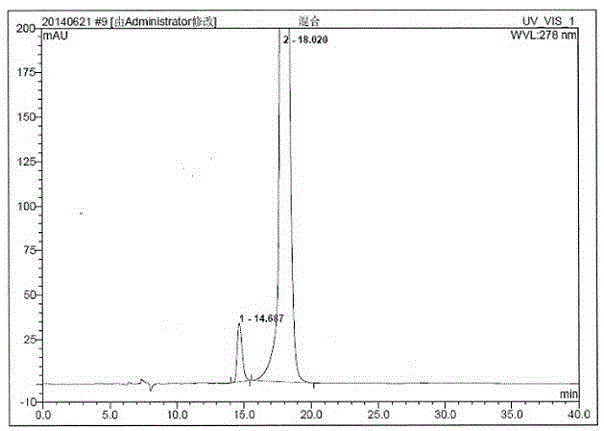
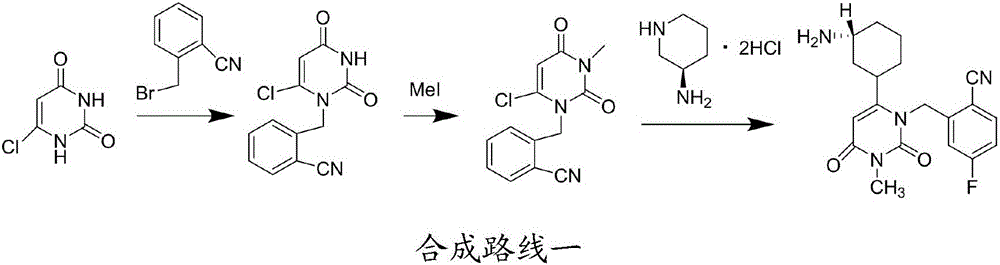
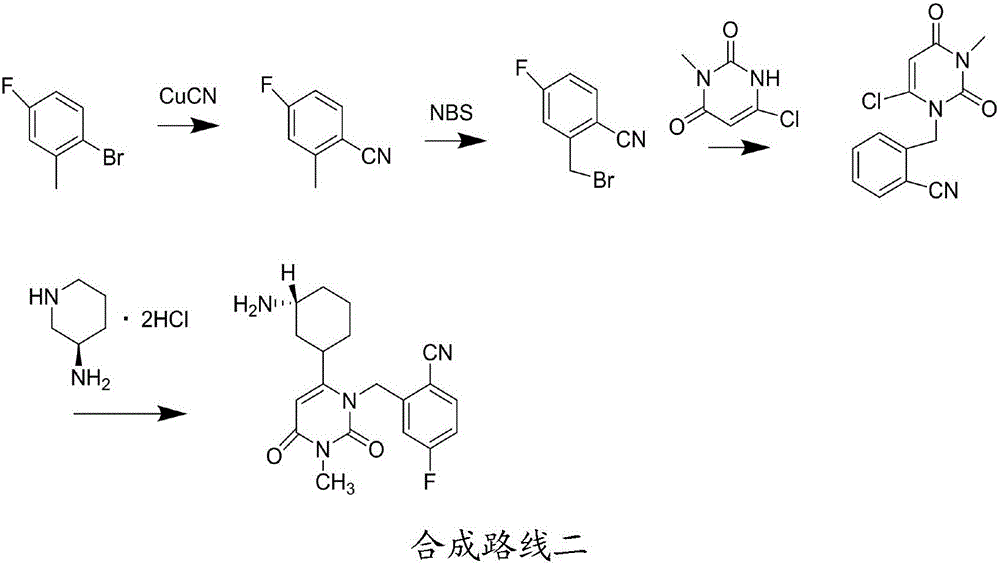
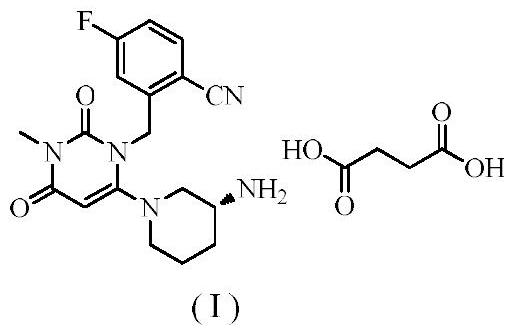
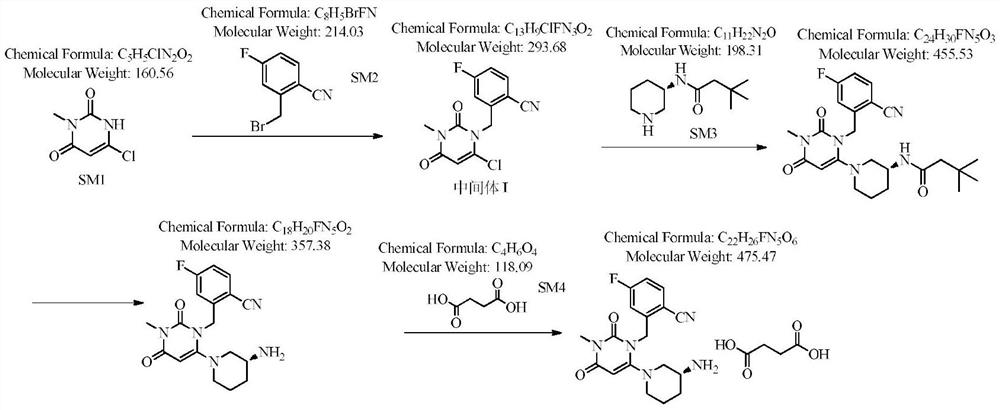
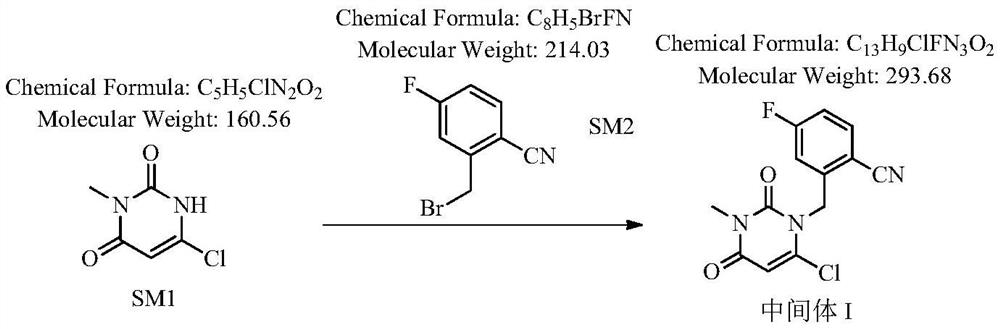
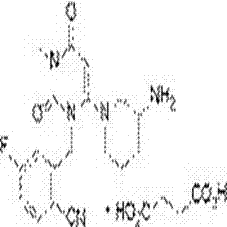
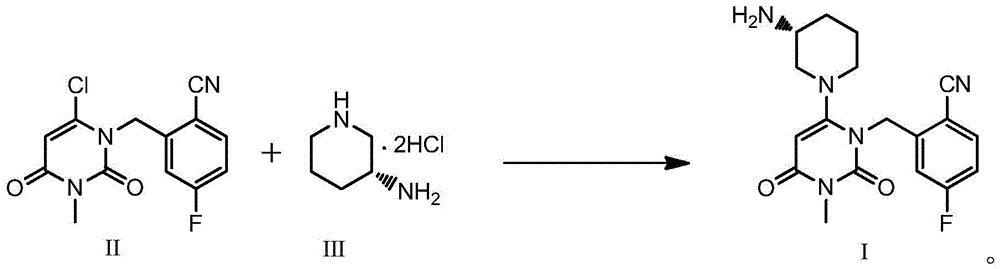Patents
Literature
57 results about "Trelagliptin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
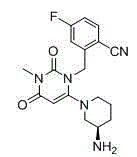
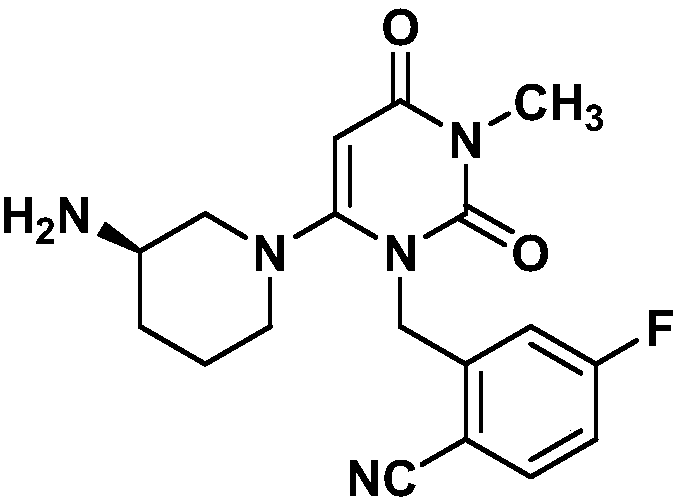
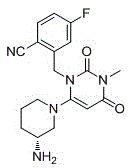
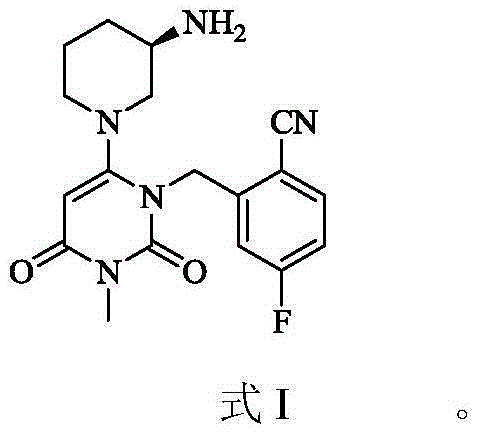
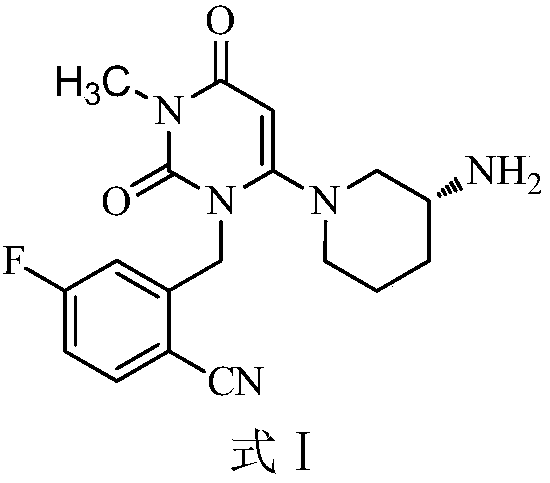
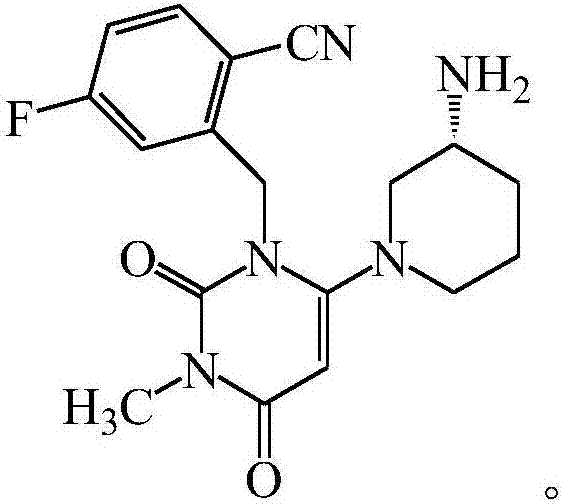
Trelagliptin (trade name Zafatek) is a pharmaceutical drug used for the treatment of type 2 diabetes (diabetes mellitus).
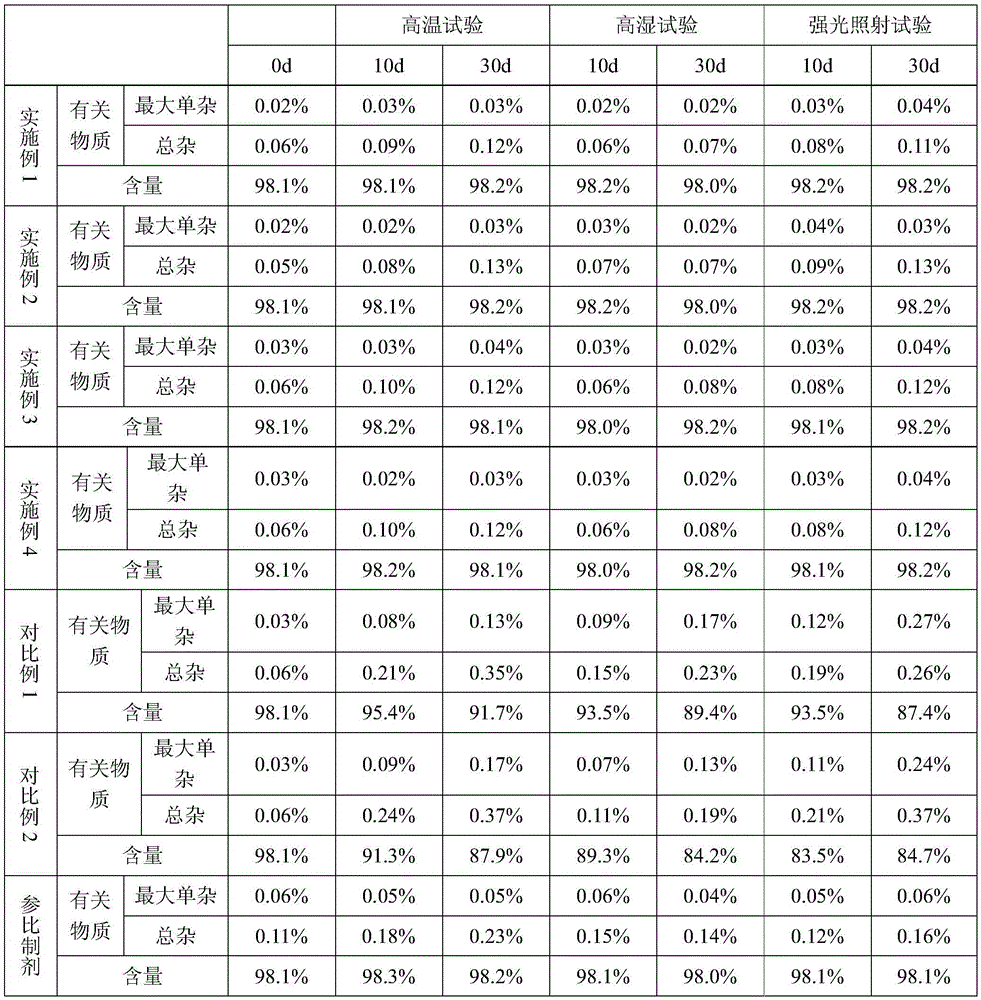
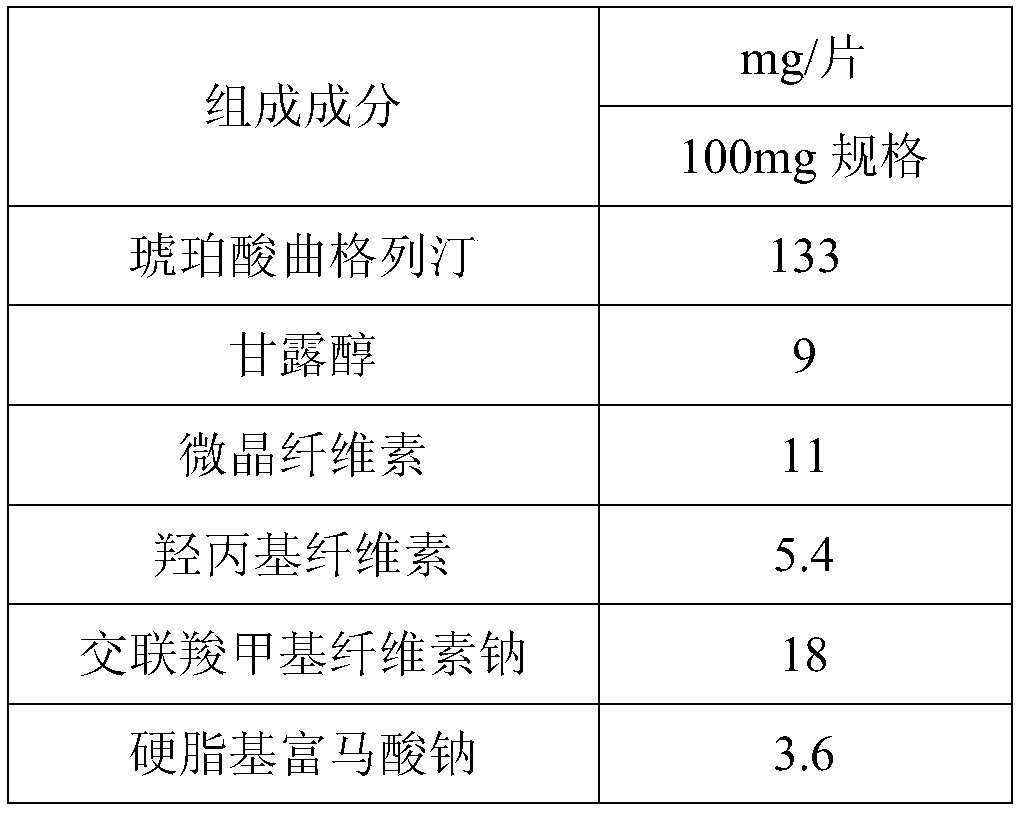
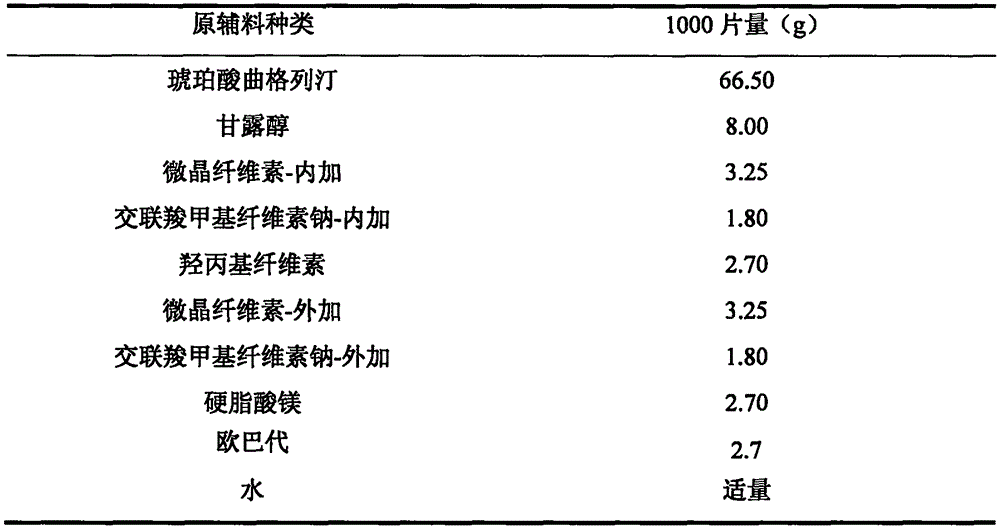
New solid forms of trelagliptin and manufacturing method and purpose thereof
InactiveCN104003975AEasy to makeEasy to prepareOrganic active ingredientsNervous disorderDiseaseDipeptidyl-Peptidase IV Inhibitors
The present invention relates to solid forms of trelagliptin, a preparation method therefor and applications thereof, and relates specifically to six new solid forms of the dipeptidyl peptidase-4 inhibitor trelagliptin and preparation methods therefor, as well as to pharmaceutical compositions comprising said solid forms of trelagliptin, and uses of same in the preparation of medicines for the treatment of diseases mediated by dipeptidyl peptidase-4.
Owner:SICHUAN HAISCO PHARMA CO LTD
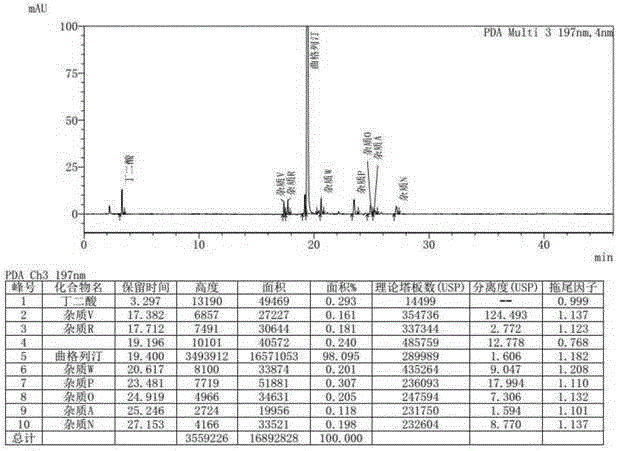
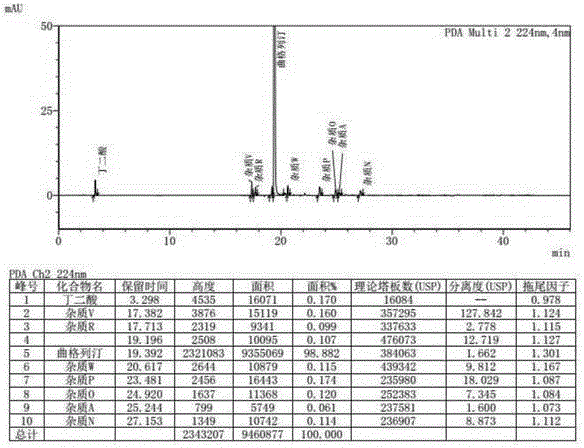
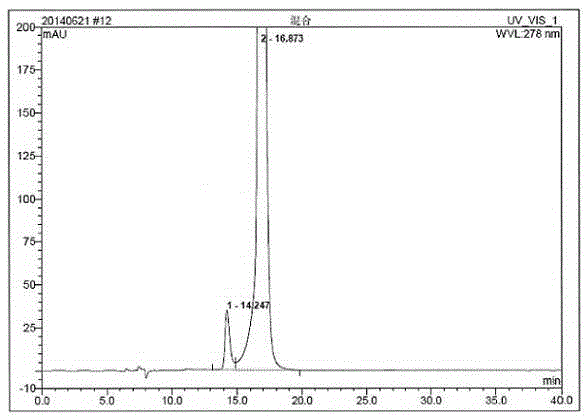
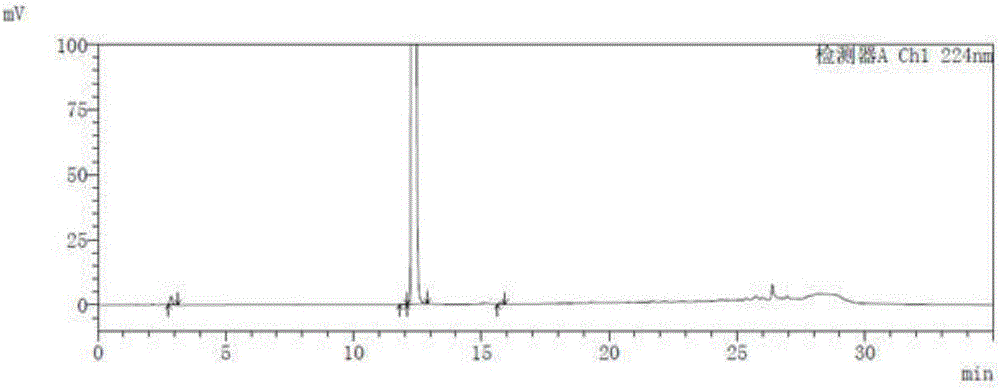
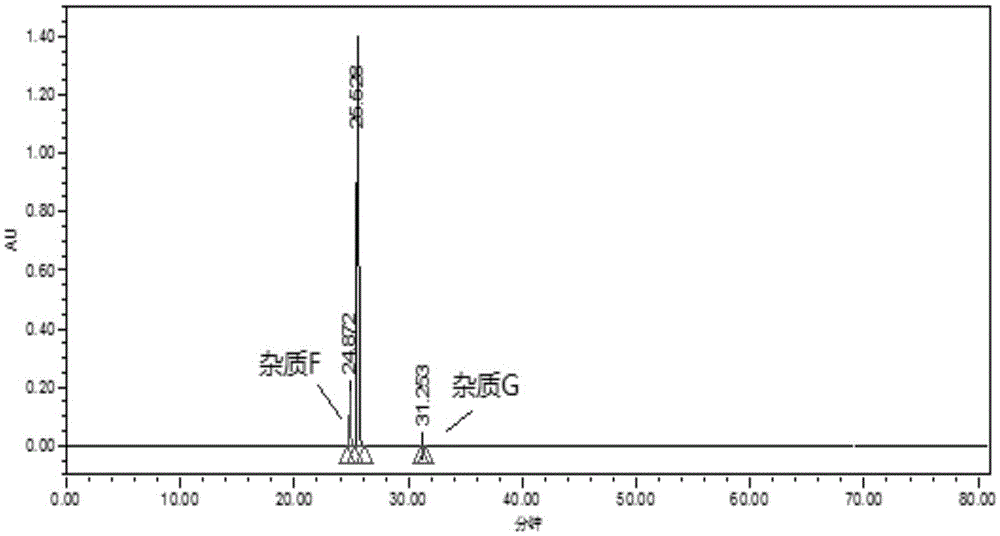
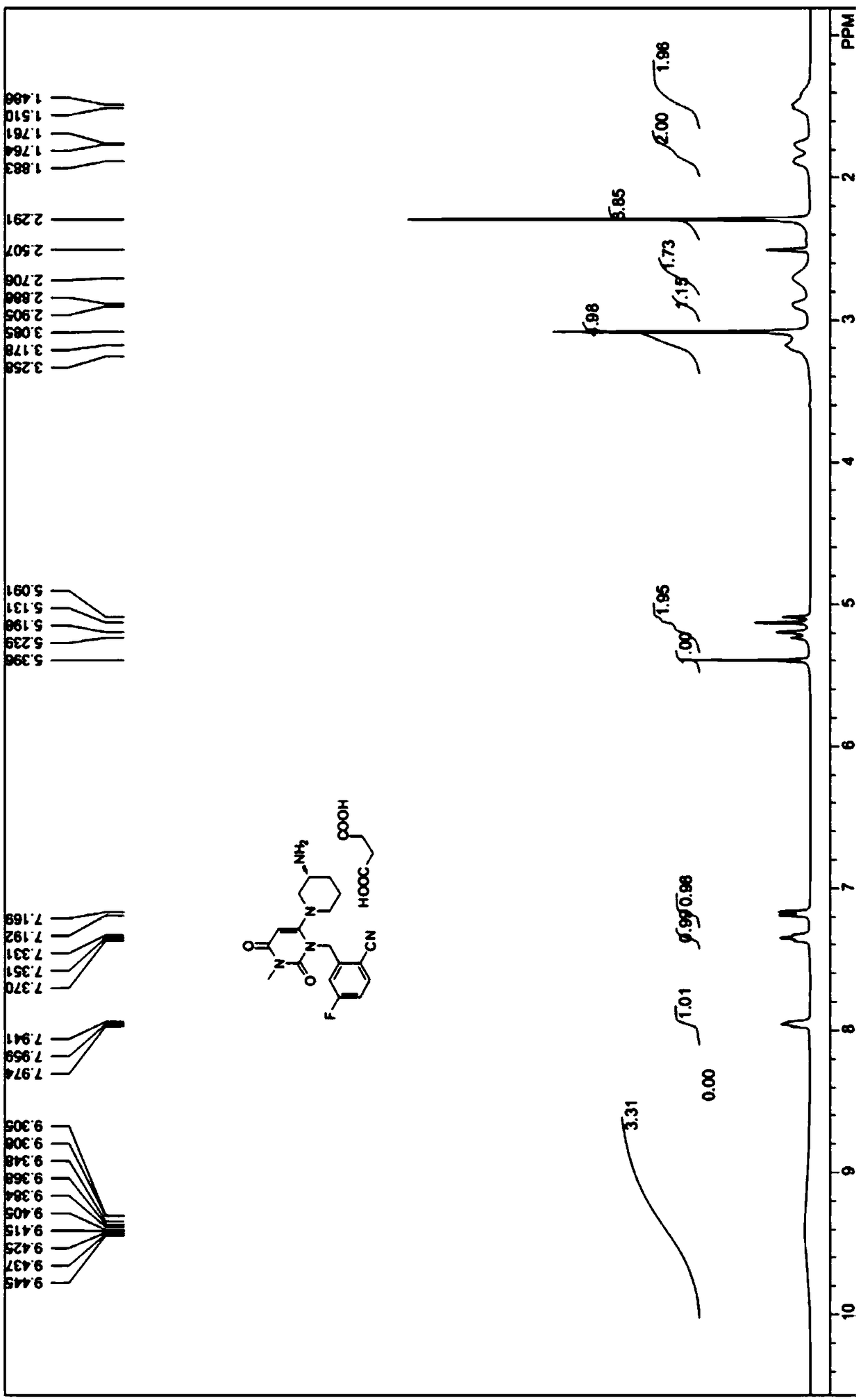
Method for measuring related substances in succinic acid Trelagliptin raw materials
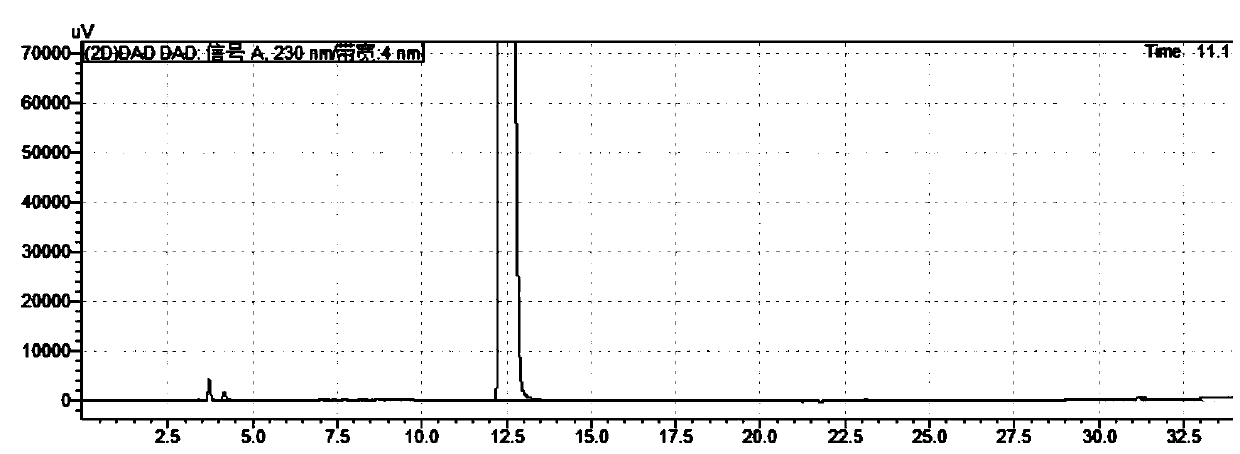
ActiveCN105699547AQuality is easy to controlEfficient separationComponent separationTrelagliptinHigh-performance liquid chromatography
The invention discloses a method for measuring related substances in succinic acid Trelagliptin raw materials and relates to the field of analytic chemistry. According to the method, high performance liquid chromatography is adopted, a sample solution is injected into a high performance liquid chromatograph, and the related substances in the succinic acid Trelagliptin raw materials are measured; according to chromatographic conditions, a 100-5C18 chromatographic column of the Kromasil company is adopted as a chromatographic column, a flowing phase A and a flowing phase B are both a mixed solution of an acid aqueous solution and an organic solvent, the sum of the volume percentage of the flowing phase A and the volume percentage of the flowing phase B is kept 100% all the time, and linear gradient elution is performed. According to the method, the 100-5C18 chromatographic column of the Kromasil company is adopted, the gradient elution program of the flowing phases is optimized, and the related substances in the succinic acid Trelagliptin raw materials can be effectively separated. The method solves the separation and measurement problem of the related substances in the succinic acid Trelagliptin raw materials, and therefore it is guaranteed that the mass of the succinic acid Trelagliptin raw materials is controllable.
Owner:中山万远新药研发有限公司
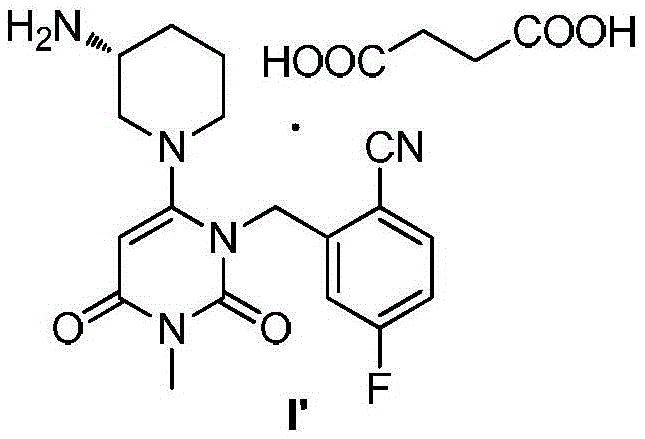
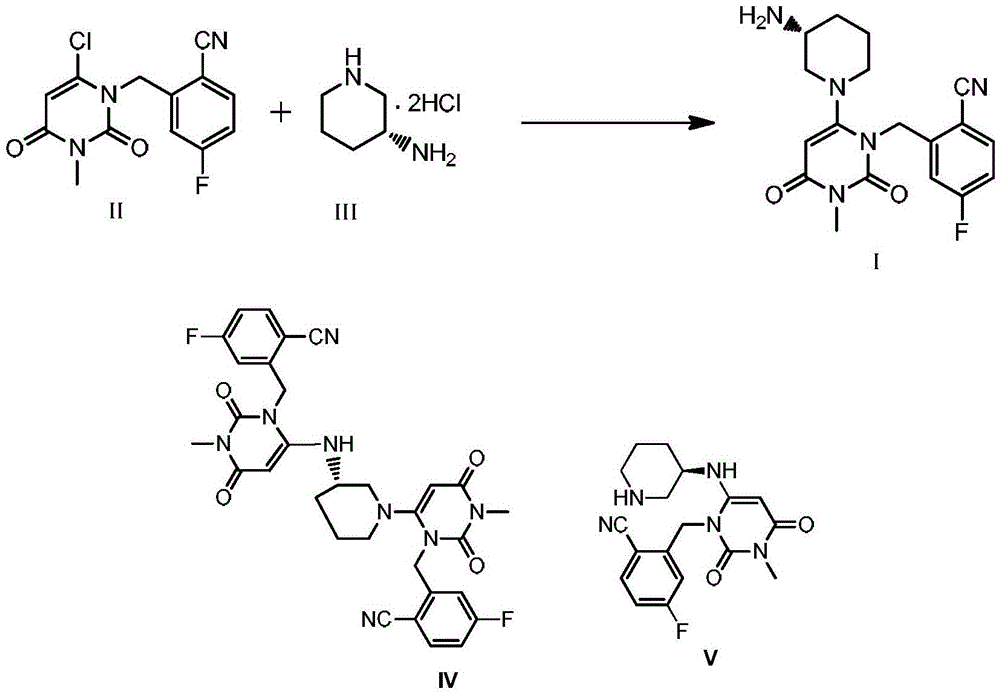
Trelagliptin and preparation method of succinate thereof
ActiveCN105669645AReduce contentSimple and safe operationOrganic chemistry methodsCarboxylic acid salt preparationOrganic solventPhosphate
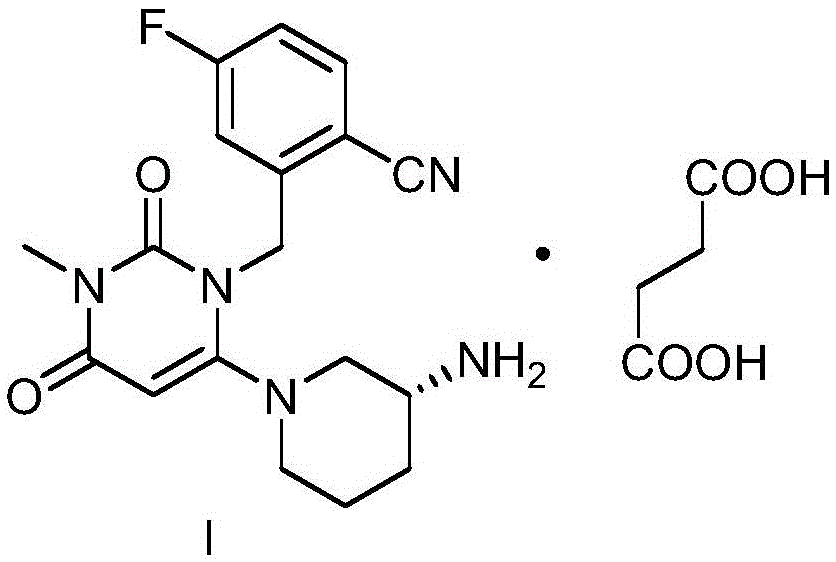
The invention discloses a trelagliptin and a preparation method of succinate thereof. The preparation method includes the following steps that a trelagliptin intermediate II and a trelagliptin intermediate III perform nucleophilic substitution reaction in an organic solvent in the presence of phosphate and a phase transfer catalyst to obtain trelagliptin I, and then trelagliptin I reacts with the succinate to produce salt. The operation process of the preparation method is simple and safe, special devices are not needed, the impurity contents of bis-substituted and primary amine substituted isomers can significantly reduced, only one-time recrystallization is needed, the purity of the obtained trelagliptin succinate I' can be above 99.5%, the impurity contents of the bis-substituted and primary amine substituted isomers are both below 0.05%, the contents of all the other impurities are all below 0.05%, the prepared trelagliptin succinate I' is high in purity, the production cost is low, and the trelagliptin and the preparation method are suitable for industrialized production.
Owner:上海新礼泰药业有限公司
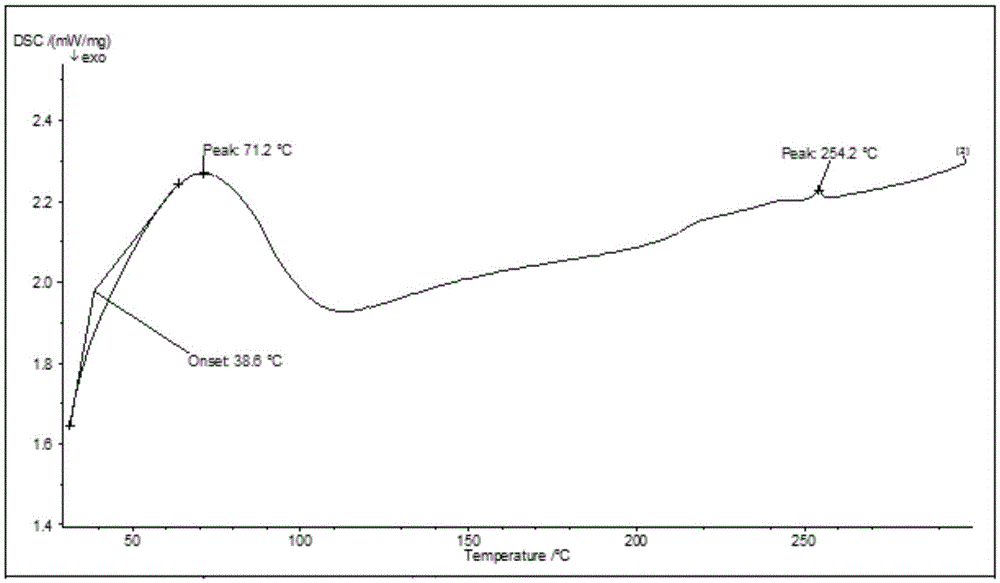
New crystal form and preparation method of highly pure trelagliptin
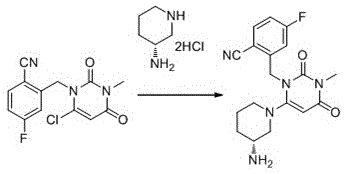
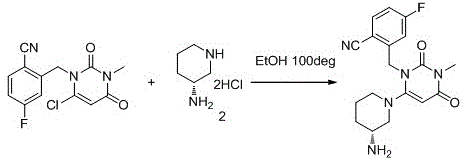
The invention provides a novel high-purity trexagliptin crystal. The high-purity trexagliptin prepared by the present invention can form a salt with an acid to obtain a high-purity trexagliptin salt, and at the same time provide a high-purity trexagliptin crystal preparation and purification crystallization process, including the following steps: Step 1: Condensation reaction, that is, compound 1 and (R)-3-aminopiperidine dihydrochloride 2 are used as raw materials, and are heated to reflux in an organic solvent and in the presence of a base to undergo a condensation reaction to obtain the crude product of trexagliptin; Step 2: Recrystallization, that is, recrystallize the crude trexagliptin obtained in step 1 in an organic solvent to obtain high-purity trexagliptin. The method of the invention has the advantages of simple operation, high yield and low cost, and is very suitable for large-scale industrial production.
Owner:SHANGHAI INST OF PHARMA IND +2
Preparation method of trelagliptin
InactiveCN104961726AControl contentAvoid it happening againOrganic chemistrySocial benefitsChemical synthesis
The invention belongs to the field of chemical synthesis of medicines, and particularly relates to a preparation method of trelagliptin. A synthesis path of the trelagliptin is shown in the specification. The trelagliptin which is protected by Boc is synthesized by (R)-3-Boc-aminopiperidines, the trelagliptin is obtained through hydrolysis deprotection, by-products produced by reaction on amidogen can be avoided, the synthesis path is novel, technological conditions are reasonable, the type and the content of impurities in the trelagliptin can be controlled effectively, a technology is easy to operate, and the yield is high. Moreover, the preparation method of the trelagliptin is suitable to be produced industrially and has high application value, high social benefit and high economic benefit.
Owner:ZHEJIANG YONGNING PHARMA
Succinic acid trelagliptin orally-disintegrating tablets and preparing method thereof
InactiveCN105496977AAromatic and refreshing tasteNo grittinessOrganic active ingredientsMetabolism disorderOrally disintegrating tabletTrelagliptin
The invention discloses succinic acid trelagliptin orally-disintegrating tablets and a preparing method thereof. The orally-disintegrating tablets are a medicine composition prepared from succinic acid trelagliptin, filler, a disintegrating agent, a wetting agent, a binding agent, a flavoring agent and a lubricating agent. Wet granulation and tabletting methods are adopted for the preparing method. The succinic acid trelagliptin orally-disintegrating tablets are simple in preparing process, low in cost, convenient to take, quick in effect taking and high in bioavailability, the medicine taking compliance of patients can be improved through the dosage form, and treatment on diseases is facilitated.
Owner:BEIJING VENTUREPHARM BIOTECH
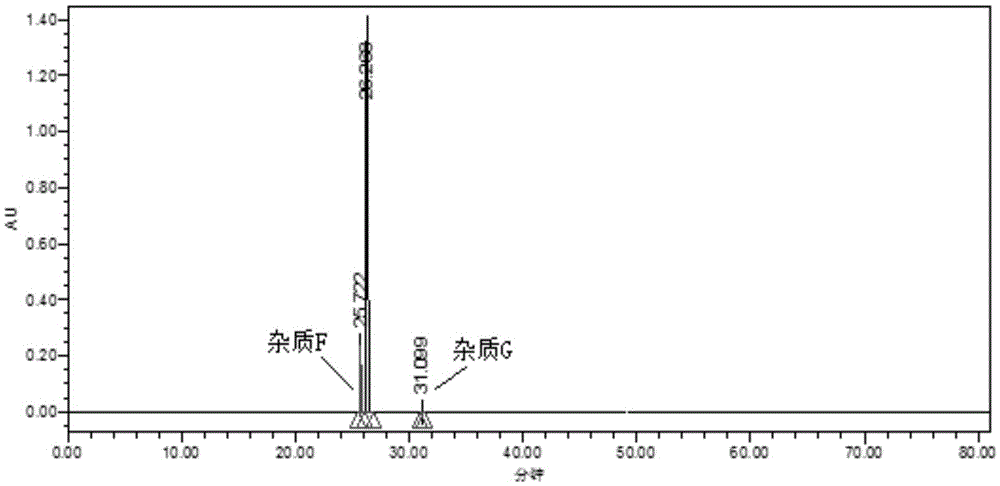
Method for determining related substances in Trelagliptin tablets
ActiveCN105738517AEfficient separationQuality is easy to controlComponent separationOrganic solventTrelagliptin
The invention provides a method for determining related substances in Trelagliptin tablets, and relates to the field of analytical chemistry. The method comprises the steps that a high-performance liquid chromatography method is adopted, a sample solution is injected into a high-performance liquid chromatographic instrument, determination of the related substances in the Trelagliptin tablets is completed, and the chromatographic conditions are that a chromatographic column takes silica gel of which the surface is provided with electric hybrid particles as a filler, a mobile phase A is an acidic aqueous solution, a mobile phase B is an organic solvent, the sum of the volume percent of the mobile phase A and the volume percent of the mobile phase B maintains 100% all the time, and linear gradient elution is conducted. According to the method for determining the related substances in the Trelagliptin tablets, an Xselect C18 chromatographic column is adopted, optimization is conducted on the mobile phase gradient elution program, and the related substances in the Trelagliptin tablets can be effectively separated. The separation determination problem of the related substances in the Trelagliptin tablets is solved, and therefore it is guaranteed that the quality of the Trelagliptin tablets is controllable.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
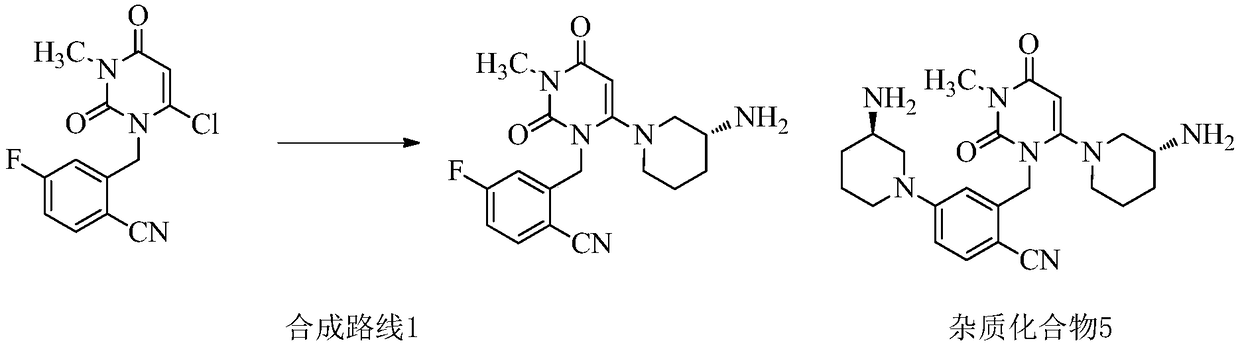
Synthetic method of trelagliptin, trelagliptin synthesized through method and trelagliptin synthesis intermediate
ActiveCN105541793AReduce usageAvoid damageCarboxylic acid nitrile preparationOrganic compound preparationHydrogenCyanide
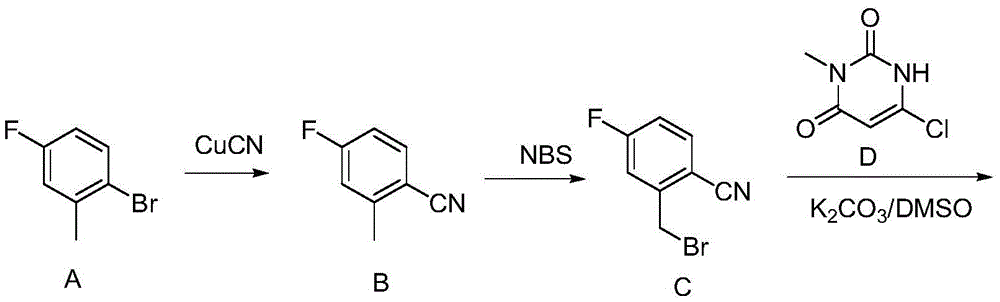
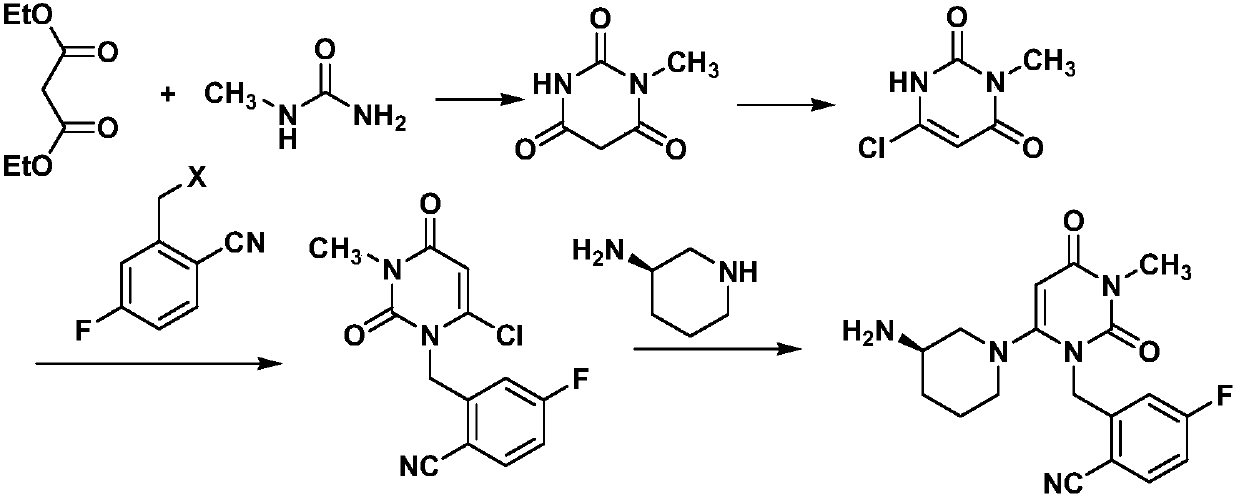
The invention discloses a synthetic method of trelagliptin, trelagliptin synthesized through the method and a trelagliptin synthesis intermediate. The synthetic method of trelagliptin comprises the following steps: 1, taking 2-hydroxymethyl-4-fluorobenzonitrile as a starting raw material, and conducting a chlorination reaction, so that 2-chloromethyl-4-fluorobenzonitrile is obtained; 2, taking the 2-chloromethyl-4-fluorobenzonitrile as an intermediate, and conducting a condensation reaction, so that 2-[(6-chlorine-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidyl)methyl]-4-fluorobenzonitrile is obtained; 3, taking the 2-[(6-chlorine-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidyl)methyl]-4-fluorobenzonitrile as an intermediate, and conducting an ammonolysis reaction, so that trelagliptin alkali is obtained. By means of the method, use of high-toxicity copper cyanide is avoided, safety of the synthesis process is improved, environment friendliness is achieved, the formed chloro intermediate is more stable, and no irritation is caused.
Owner:HEBEI GUOLONG PHARMA CO LTD
Method for preparing trelagliptin
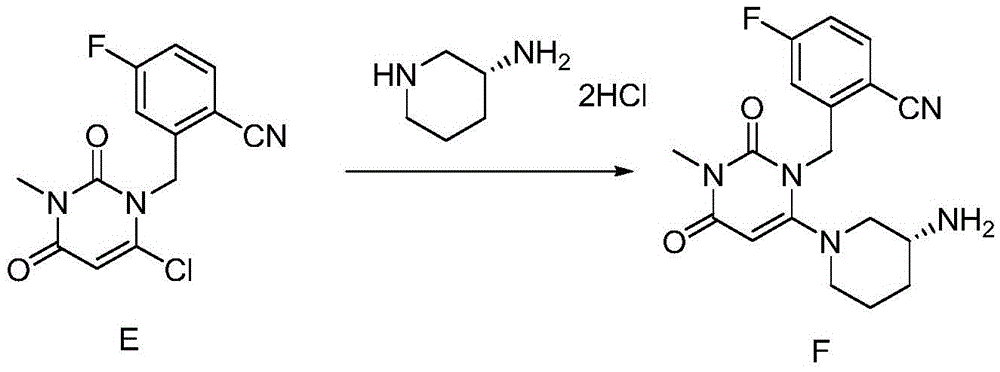
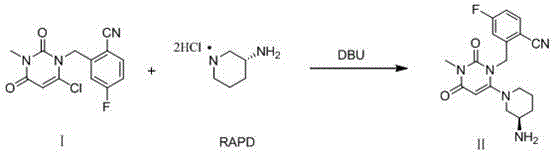
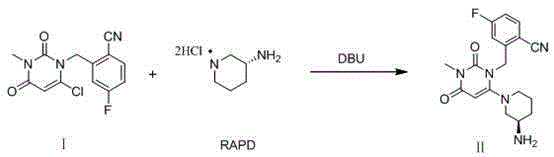
The invention relates to a method for preparing trelagliptin. The method comprises the following steps: 1) in a reaction solvent, adding 2-(6-chlorine-3-methyl-2,4-dione-3,4-dihydropyrimidine-dihydro-1-methyl)-4-F-benzonitrile into R-3-piperidinamine dihydrochloride, dropwise adding 1,8-diazabicyclo[5,4,0]-undec-7-ene in ice bath, at temperature of 15-25 DEG C, insulating the materials for reacting for 2 hours, 2) using hydrochloric acid to adjust a reaction solution to acidity, 3) adding an organic solvent and adjusting a pH value of a water phase to alkalescence, extracting the material to obtain a trelagliptin crude product, and 4) using a solvent for recrystallization to obtain trelagliptin. The obtained trelagliptin can effectively control chromatographic purity being no less than 99.0% and yield being no less than 75%, process is simple, and cost is low.
Owner:CHONGQING PHARMA RES INST
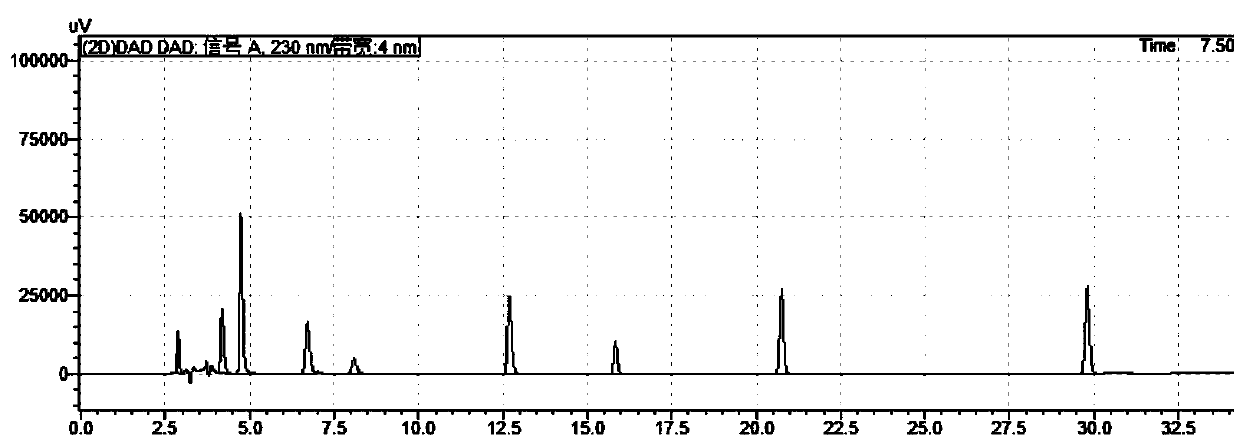
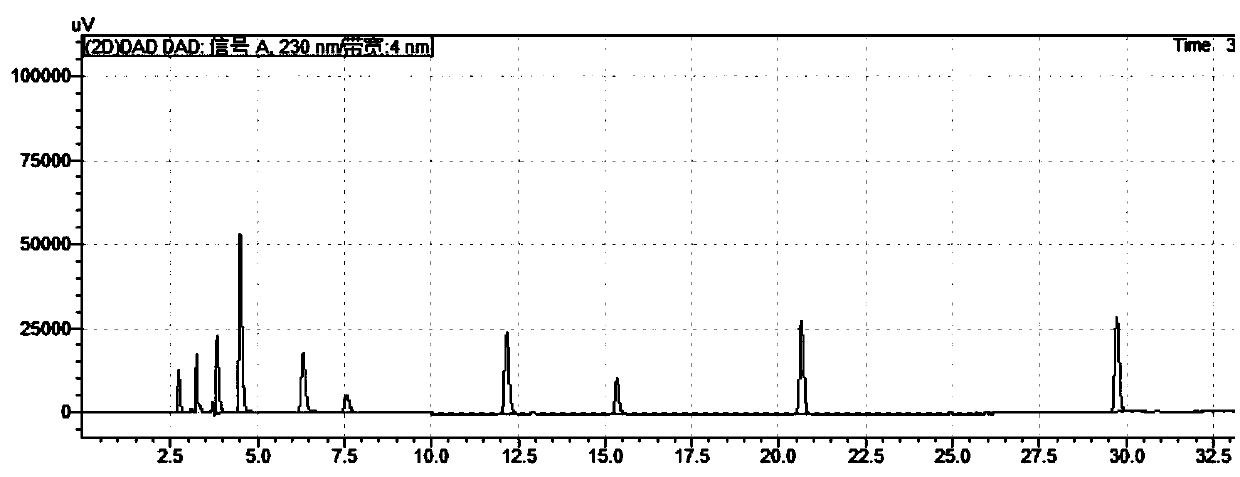
Method for separating and analyzing trelagliptin succinate and preparation related substances thereof
InactiveCN106290596AEfficient separationGood elution separation effectComponent separationStationary phaseOrganic solvent
The invention discloses a method for separating and analyzing trelagliptin succinate and preparation related substances thereof. According to the method, the chromatographic conditions comprise that an octadecyl bonded silica gel is adopted as a stationary phase, a buffer liquid is adopted as a mobile phase A and an organic solvent is adopted as a mobile phase B to carry out gradient elution, and the gradient elution conditions comprise that the mobile phase A is 85-50 (V%) and the mobile phase B is 15-50 (V%) at the time of 0-10 min, the mobile phase A is 50 (V%) and the mobile phase B is 50 (V%) at the time of 25-30 min, and the mobile phase A is 85-50 (V%) and the mobile phase B is 15-50 (V%) at the time of 31-35 min; the sample solution preparation comprises that the sample to be detected is prepared into the sample solution by using the mobile phase A; and separating and analyzing are performed, wherein the sample solution is injected into a high performance liquid chromatography so as to complete the detection of the trelagliptin succinate and the preparation related substances thereof. With the method of the present invention, the trelagliptin and the related substances can be rapidly and effectively separated under the same chromatographic conditions. The detection method of the present invention has advantages of high specificity, high precision, strong accuracy, convenient operation, and effective control of drug quality.
Owner:WATERSTONE PHARMA WUHAN
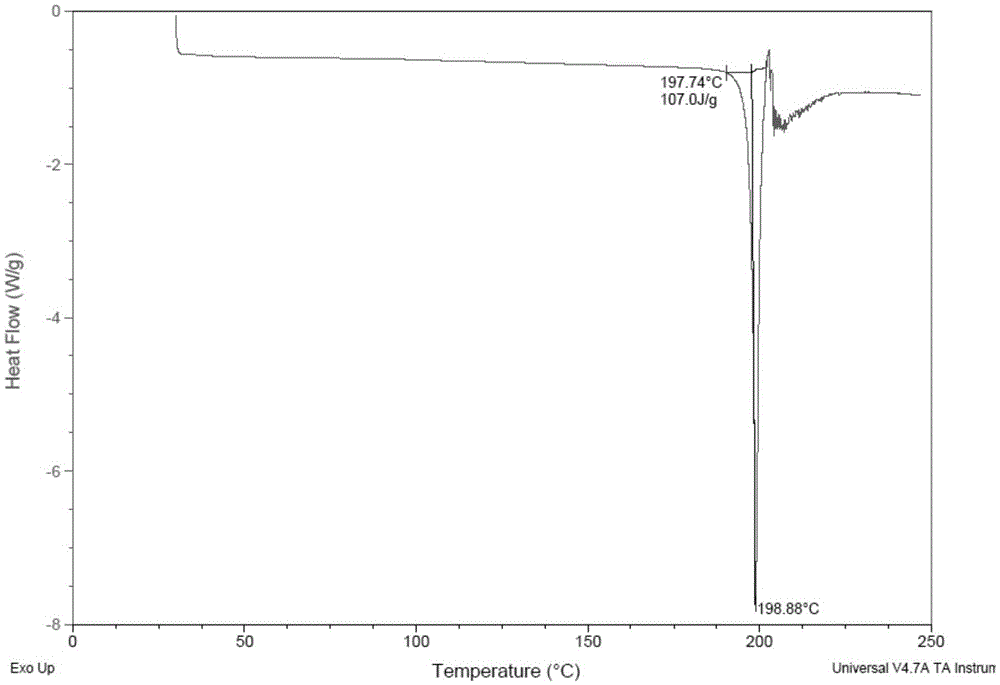
Trelagliptin succinate crystal form A preparation method
The present invention relates to a trelagliptin succinate crystal form A preparation method, and belongs to the technical field of the chemistry. The technical scheme comprises: preparing a trelagliptin fine product, and preparing a trelagliptin succinate bulk drug. The invention provides the trelagliptin succinate crystal form A preparation method suitable for industrial production, wherein the purity of the product prepared through the method is more than 99.95%; and the used single solvent anhydrous ethanol can be recovered and applied, is the optimal solvent for preparing the trelagliptin succinate crystal form A, is the bulk drug refining solvent recommended in ICH, and has characteristics of low prices and environmental protection.
Owner:WEIHAI DISU PHARMA CO LTD +1
Succinic acid trelagliptin raw medicine and HPLC analysis method of preparation enantiomer thereof
InactiveCN106546689AEffectively separate and measureHigh sensitivityComponent separationAlcoholEnantiomer
The invention belongs to the technical field of medicine analysis and particularly relates to a succinic acid trelagliptin raw medicine and a testing method of a preparation enantiomer thereof. A chiral chromatographic column with the silica gel surface coated with ADMPC as filler is used, and the uccinic acid trelagliptin enantiomer is measured with a lower alcohol mixed solution and lower aliphatic amine or lower alcohol amine as mobile phases. The method is easy and quick to operate, good in reproducibility and high in sensitivity.
Owner:JIANGSU CAREFREE PHARM CO LTD +1
Preparation method of succinic acid Trelagliptin tablets
ActiveCN105476974AQuality improvementGood in vitro dissolution behaviorOrganic active ingredientsMetabolism disorderCarboxymethyl celluloseTrelagliptin
The invention provides a preparation method of succinic acid Trelagliptin tablets. The method includes: taking succinic acid Trelagliptin, adding sorbitol, microcrystalline cellulose and crosslinking sodium carboxymethyl cellulose, mixing, placing mixture under a condition with certain temperature and relative humidity, moving into a vacuum kettle, rising the temperature under a vacuum condition, adding binder, performing wet granulation, drying obtained granules in a fluidized bed granulation coating machine to obtain dried granules, adding lubricant into the dry granules, mixing, tableting, and coating to obtain the succinic acid Trelagliptin tablets. The preparation method has the advantages that the succinic acid Trelagliptin tablets prepared by the method is stable in quality and good in in-vitro dissolution performance, the method is simple in production process, and large-scale industrial production can be achieved.
Owner:HONG KONG JOWA & HUAYUAN GRP CHUZHOU PHARMA CO LTD
Method for determining trelagliptin related substances
The invention relates to the field of chemical analysis, and particularly relates to a method for determining trelagliptin related substances. The method for determining trelagliptin related substances comprises the following steps: using octadecylsilane bonded silica gel as a chromatographic column filler and sampling a test article solution; then carrying out elution by using an acid solution asa flow phase A and an organic solvent as a flow phase B and carrying out detection. The octadecylsilane bonded silica gel and the acid solution are selected as the flow phase A and the organic solvent is selected as the flow phase B, so that the trelagliptin related substances are conveniently separated and eluted, and impurities of trelagliptin is fully revealed, the trelagliptin related substances can be quickly detected, and the security of the product is improved. The method disclosed by the invention has the advantages of science and reliability, and moreover, the trelagliptin related substances can be controlled.
Owner:NANJING VARSAL MEDICINE TECH DEV +1
Preparation method for trelagliptin
InactiveCN107698560AShort reaction timeShort reaction cycleOrganic chemistryAfter treatmentTrelagliptin
The invention discloses a preparation method for trelagliptin and belongs to the field of organic synthesis. The preparation method comprises the following steps: (1) taking methylurea and diethyl malonate as initial raw materials and performing cyclization and chlorination reaction, thereby acquiring 3-methyl-6-chlorouracil; (2) acquiring 2-(6-chlorine-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidine-1-methyl)-4-fluorobenzonitrile from the nucleophilic substitution reaction of 3-methyl-6-chlorouracil and 2-bromine methyl-4-fluorobenzonitrile; (3) generating a target compound (R)-2-((6-(3-aminopiperidines-1-group)-3-methyl-2,4-dioxo-3,4-dihydropyrimidine-1(2H)-group) methyl-4-fluorobenzonitrile (trelagliptin) through the reaction of 2-(6-chlorine-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidine-1-methyl)-4-fluorobenzonitrile and (R)-3-aminopiperidine. The preparation method has the characteristics of low-cost and easily acquired initial raw materials, convenient after-treatment, mild condition, operation convenience, and the like.
Owner:SHIJIAZHUANG DUEN MEDICINE SCI & TECHCO
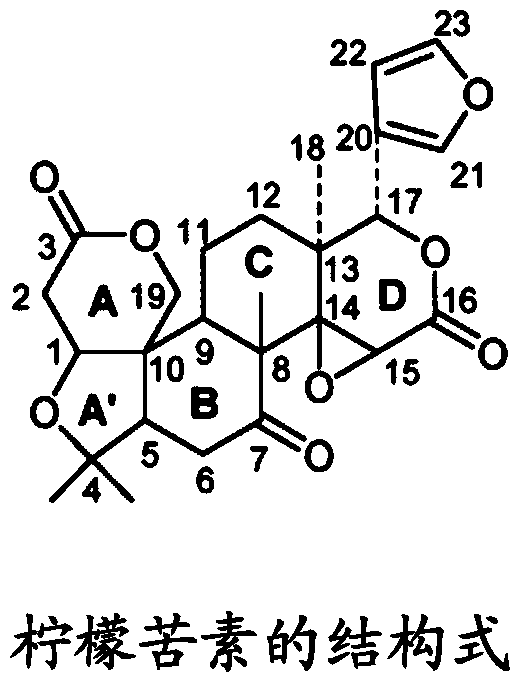
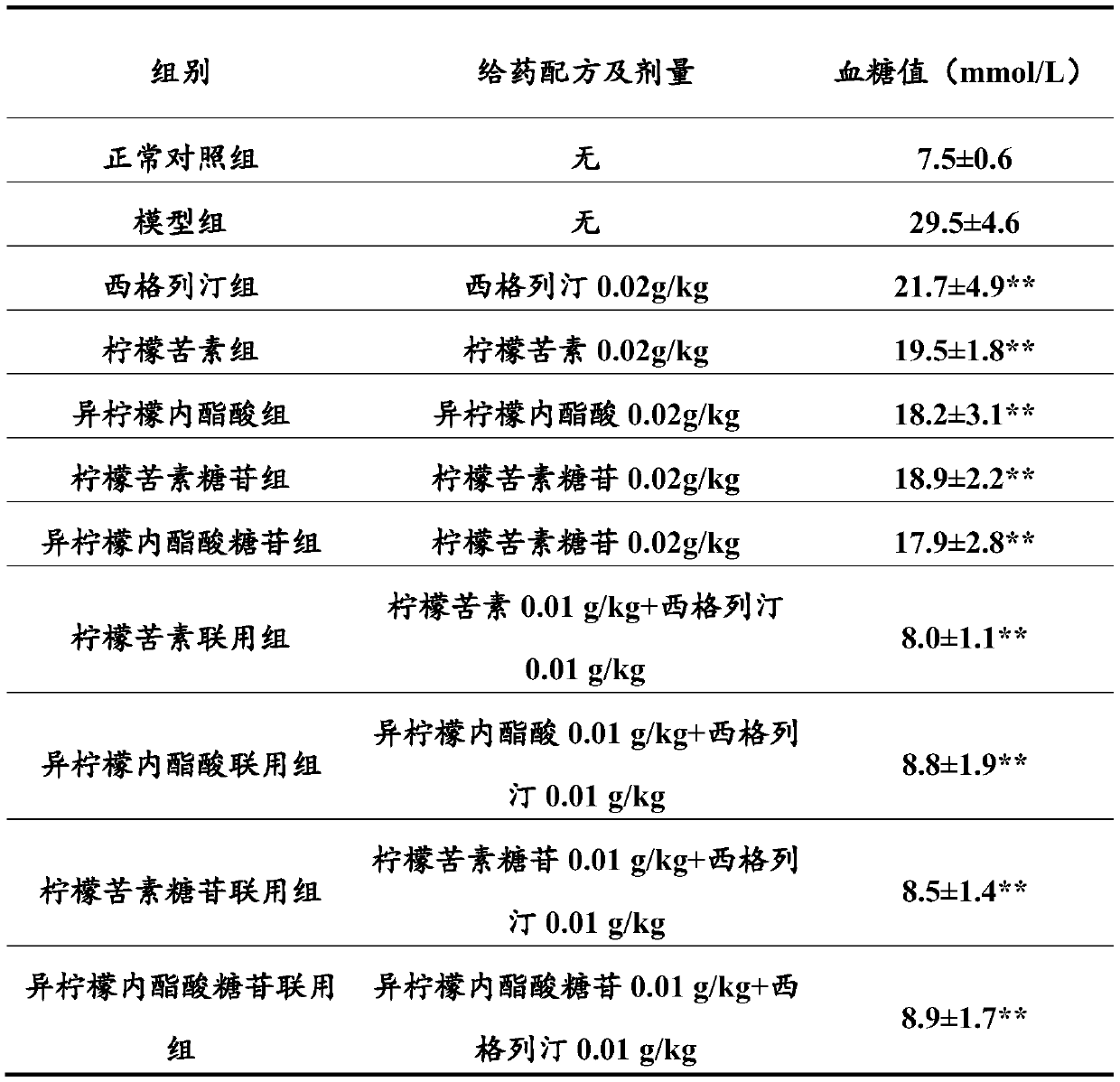
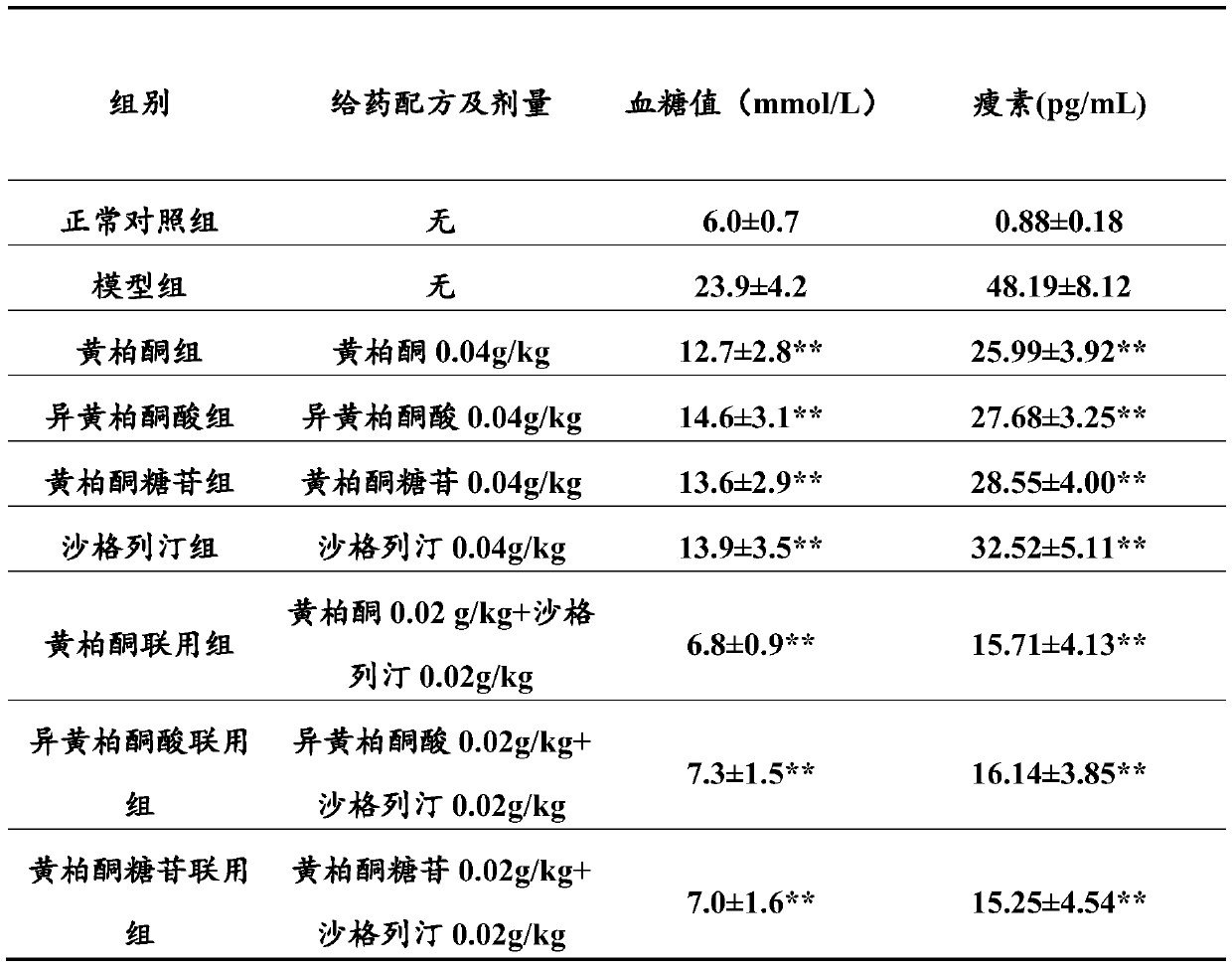
Combination product containing limonin compound and DPP-4 inhibitors
The invention relates to a combination product of a limonin compound, pharmaceutically-acceptable derivatives, esters, stereoisomers, salts or prodrugs thereof and dipeptidyl peptidase-4(DPP-4) inhibitors such as sitagliptin, saxagliptin, vildagliptin, linagliptin, alogliptin, trelagliptin and omarigliptin. The invention further relates to application of the combination product in treating and / orpreventing diseases related to diabetes.
Owner:NATURAL MEDICINE INST OF ZHEJIANG YANGSHENGTANG
Trelagliptin preparation method
ActiveCN106008459AHigh selectivitySimple post-processingOrganic chemistryOrganic solventTrelagliptin
The invention discloses a Trelagliptin preparation method. The method comprises that a compound shown in the formula 6 and a compound shown in the formula 5 undergo a condensation reaction in the presence of an organic solvent and an alkali to produce a compound shown in the formula 4, a compound shown in the formula 2 is prepared through halogenations and azidation, or acid anhydride formation and azidation, or direct azidation, or esterification, hydrazide formation and azidation, or esterification and azidation, and Trelagliptin is prepared through a rearrangement hydrolysis reaction. The method has the advantages of mild reaction conditions, high conversion rate, less impurities, low cost and high finished product purity and is suitable for industrial production.
Owner:山东四环药业股份有限公司
A method for determining related substances in trexagliptin tablets
ActiveCN105738517BEfficient separationQuality is easy to controlComponent separationTrelagliptinGradient elution
The invention provides a method for determining related substances in Trelagliptin tablets, and relates to the field of analytical chemistry. The method comprises the steps that a high-performance liquid chromatography method is adopted, a sample solution is injected into a high-performance liquid chromatographic instrument, determination of the related substances in the Trelagliptin tablets is completed, and the chromatographic conditions are that a chromatographic column takes silica gel of which the surface is provided with electric hybrid particles as a filler, a mobile phase A is an acidic aqueous solution, a mobile phase B is an organic solvent, the sum of the volume percent of the mobile phase A and the volume percent of the mobile phase B maintains 100% all the time, and linear gradient elution is conducted. According to the method for determining the related substances in the Trelagliptin tablets, an Xselect C18 chromatographic column is adopted, optimization is conducted on the mobile phase gradient elution program, and the related substances in the Trelagliptin tablets can be effectively separated. The separation determination problem of the related substances in the Trelagliptin tablets is solved, and therefore it is guaranteed that the quality of the Trelagliptin tablets is controllable.
Owner:ZHONGSHAN WANHAN PHARM CO LTD
Trelagliptin clathrate, preparation method and application thereof
InactiveCN105561326ASimple production processImprove quality stabilityOrganic active ingredientsPowder deliveryDiabetes mellitusSolubility
The invention provides a trelagliptin clathrate, which is composed of trelagliptin and cyclodextrin or derivatives of cyclodextrin, and the weight ratio of trelagliptin to cyclodextrin or derivatives of cyclodextrin is 1:1-1:30. The invention also provides a preparation method of the trelagliptin clathrate. According to the preparation method, cyclodextrin or derivatives of cyclodextrin is taken as the subject, trelagliptin is taken as the object, and through the clathration in the presence of a solvent, trelagliptin is included or embedded into cyclodextrin or derivatives of cyclodextrin to from the clathrate. The invention also provides an application of the trelagliptin clathrate in the preparation of drugs for treating diabetes. The trelagliptin clathrate is a new solid state of trelagliptin, the problems of multiple crystal forms or mixed crystals of trelagliptin are solved, thus the production technology is simplified, at the same time, trelagliptin clathrate has the advantages of increased water solubility and good stability, moreover, the preparation method has the advantages of simple and convenient operation, little material loss, and low cost.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD
Preparation method of Trelagliptin
The invention belongs to the technical field of medicinal chemistry and particularly relates to a preparation method of Trelagliptin. The equation is shown in the specification. In the method, 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydropyrimidine-1(2H)-methyl)-4-fluorobenzonitrile and (R)-3-aminopiperidine dihydrochloride are subjected to a normal-pressure reaction in a single organic solvent or a mixed solvent under the alkaline condition of an acid-binding agent, and then a Trelagliptin compound is obtained. By means of the method, a high-pressure reaction is avoided, and thus reaction safety is greatly enhanced. The reaction condition of the method is mild and easy to control, and the method can be applied to industrial production and preparation of Trelagliptin.
Owner:SHANGHAI YUANZHI BIOMEDICAL SCI & TECH CO LTD
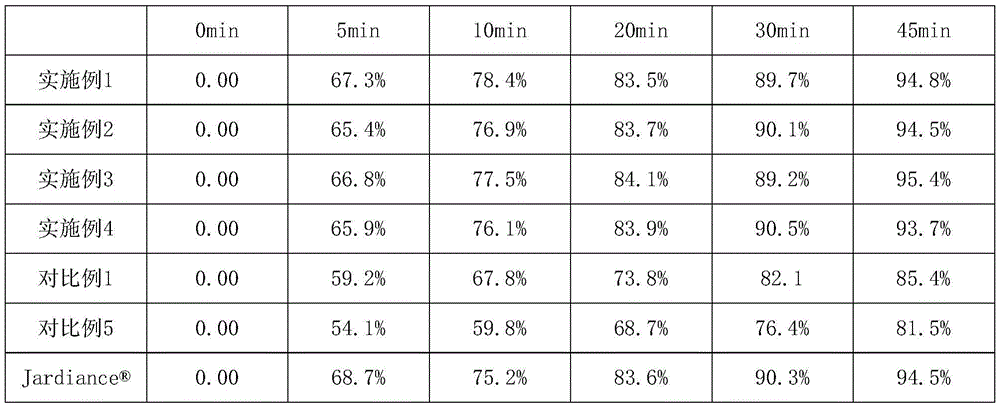
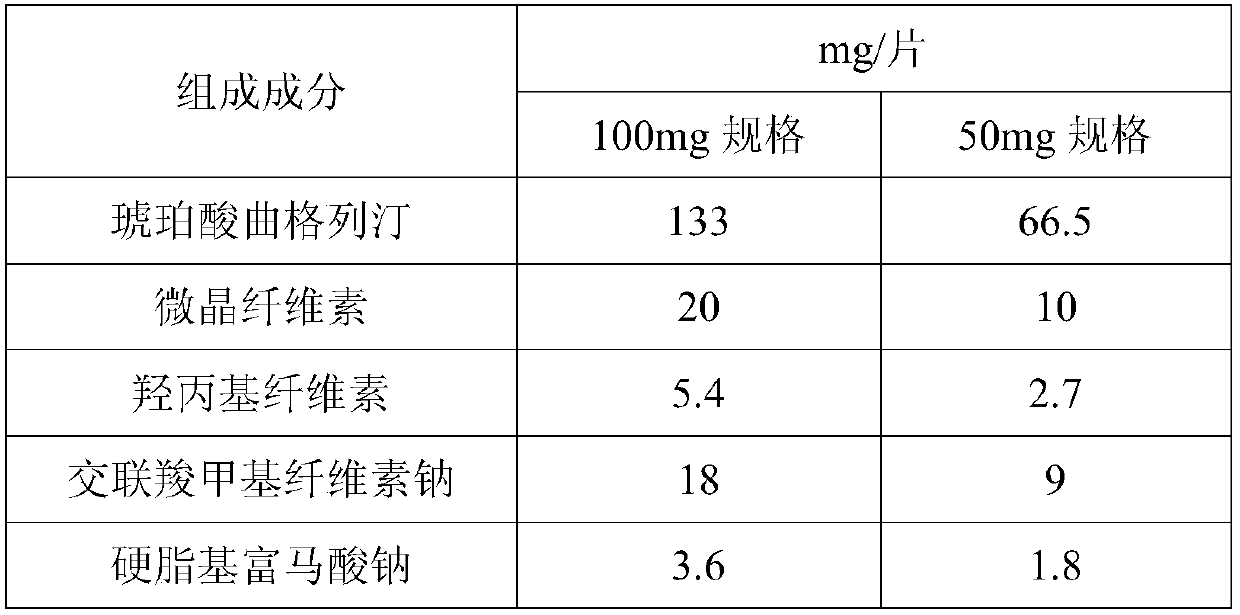
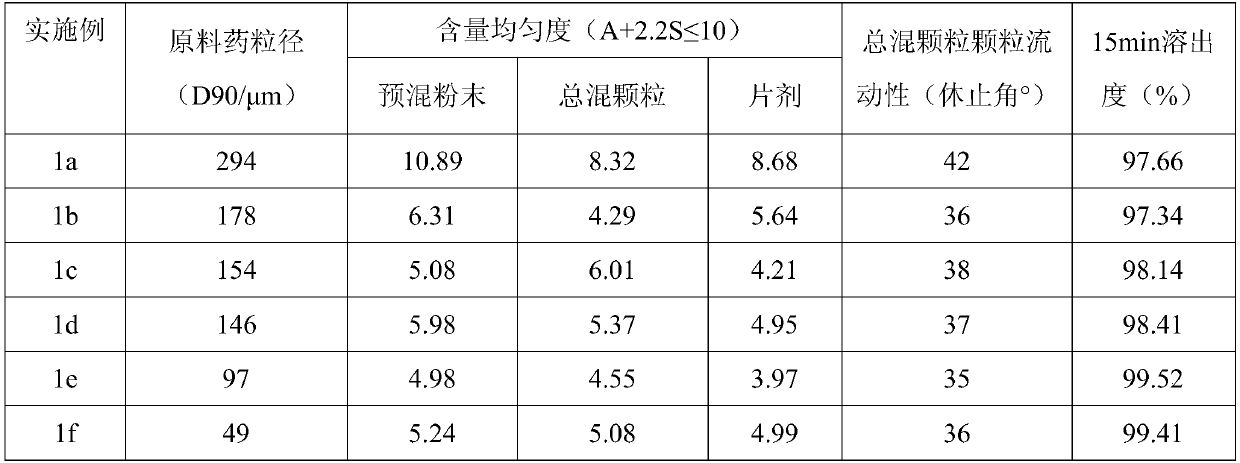
Succinic acid trelagliptin solid preparation and preparation method thereof
ActiveCN105596341AImprove liquidityGood compressibilityOrganic active ingredientsMetabolism disorderTrelagliptinCompressibility
The invention provides a succinic acid trelagliptin solid preparation and a preparation method thereof. The succinic acid trelagliptin solid preparation comprises 13-84wt% of succinic acid trelagliptin and 10-84wt% of mixture of mannitol and pregelatinized starch. The preparation method of the solid preparation is wet granulation and comprises the following concrete processes: (1) uniformly mixing succinic acid trelagliptin, a diluting agent and a disintegrating agent, then adding a wetting agent for wet granulation, and drying to obtain a mixture I; and (2) adding a lubricating agent into the mixture I, totally blending, and tabletting. According to the solid preparation, by wet granulation of the raw materials and auxiliary materials, the problems of loose and light properties and poor liquidity and compressibility of the raw materials are solved; and by adopting the specific auxiliary materials, the generation of impurities of the raw materials under hygrothermal conditions is avoided.
Owner:SHIJIAZHUANG NO 4 PHARMA
Trelagliptin succinate solid preparation and preparation method thereof
PendingCN110063942AAvoid it happening againGuaranteed stabilityOrganic active ingredientsMetabolism disorderFluidized bed dryingAdhesive
The invention discloses a trelagliptin succinate solid preparation and a preparation method thereof. The method comprises the following steps that by mass, 50-80 parts of trelagliptin succinate, 5-20parts of a filling agent, 1-10 parts of an adhesive, 3-15 parts of a disintegrating agent and 0-5 parts of a lubricant are prepared; trelagliptin succinate, the filling agent and the adhesive are mixed proportionally, and pre-mixed powder is obtained; then, water is added into the pre-mixed powder for granulation to obtain medicated granules; the medicated granules are dried by a fluidized bed fordrying, and dried medicated granules are obtained; the disintegrating agent and the lubricant are added into the dried medicated granules, and uniform mixing is performed to obtain total mixed granules; then, pressing and tableting are performed to obtain trelagliptin tablet cores. The method is simple and feasible in technology, the dissolution of the obtained solid preparation meets the requirement of a quick release agent, the tablet wright difference is small, and stability is good.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Tablet containing trelagliptin or trelagliptin succinate and preparation method of tablet
The invention relates to a tablet containing trelagliptin or trelagliptin succinate and a preparation method of the tablet, and belongs to the technical field of medicine preparations. The tablet can be singly used or jointly used together with other oral hypoglycemic agents, and is suitable for a patient with Type II diabetics. The tablet contains the trelagliptin or the trelagliptin succinate, a thinner, an adhesive, a disintegrant and a lubricant. The preparation method of the tablet containing the trelagliptin or the trelagliptin succinate comprises the following steps of (1) obtaining of medicine particles: uniformly mixing the trelagliptin or the trelagliptin succinate, the disintegrant and / or the thinner, adding a proper amount of adhesive, and granulating, so as to obtain the medicine particles; (2) obtaining of tabletting particles: uniformly mixing the medicine particles, the lubricant, the disintegrant and / or the thinner, so as to obtain the tabletting particles; (3) compression and forming: compressing and forming the tabletting particles by proper equipment. The tablet containing the trelagliptin or the trelagliptin succinate has the advantages that the dissolvability is good, the stability is good, the technology operation is simple and convenient, and the like; the technology is simple, the repeatability is good, and the tablet is especially suitable for industrial production.
Owner:BEIJING SHENLANHAI BIO PHARM TECH
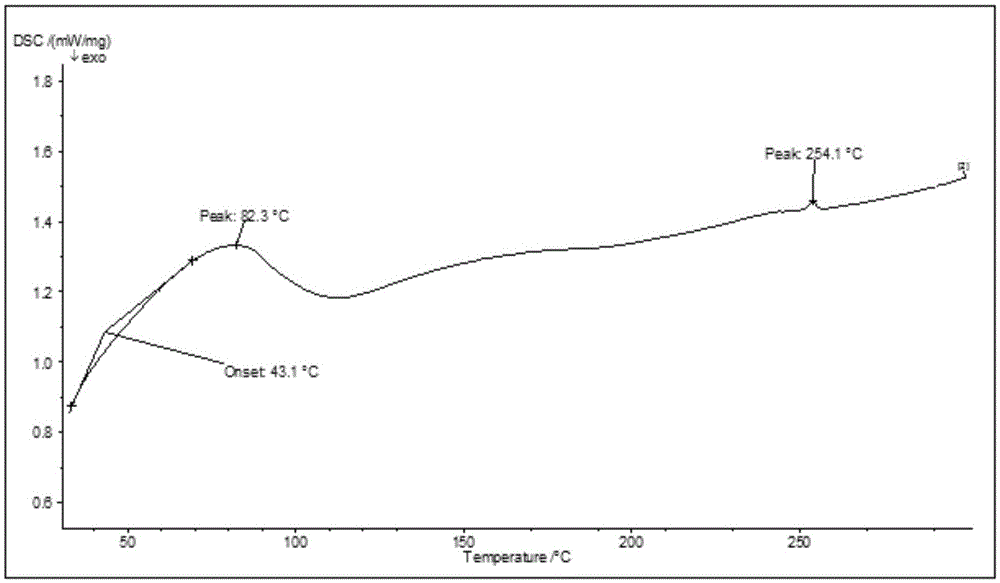
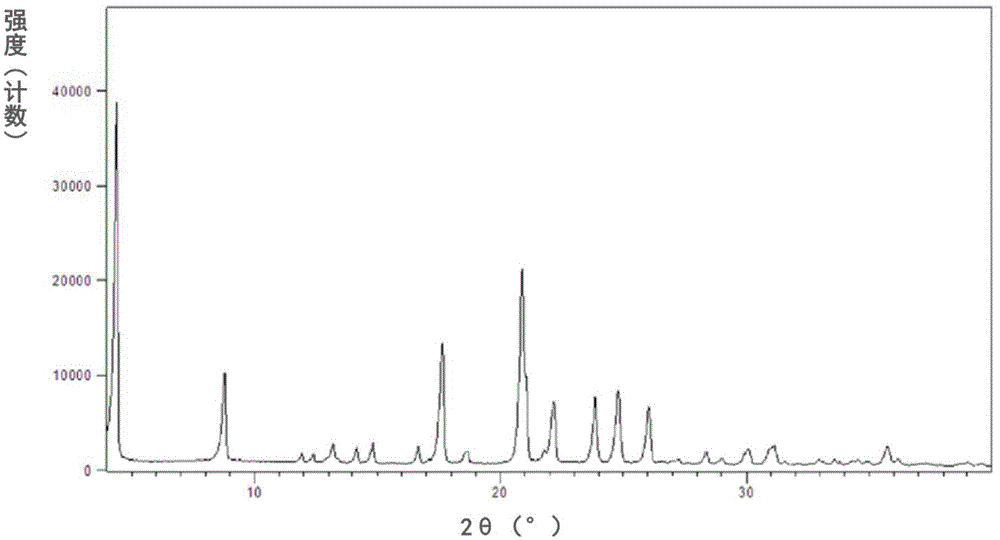
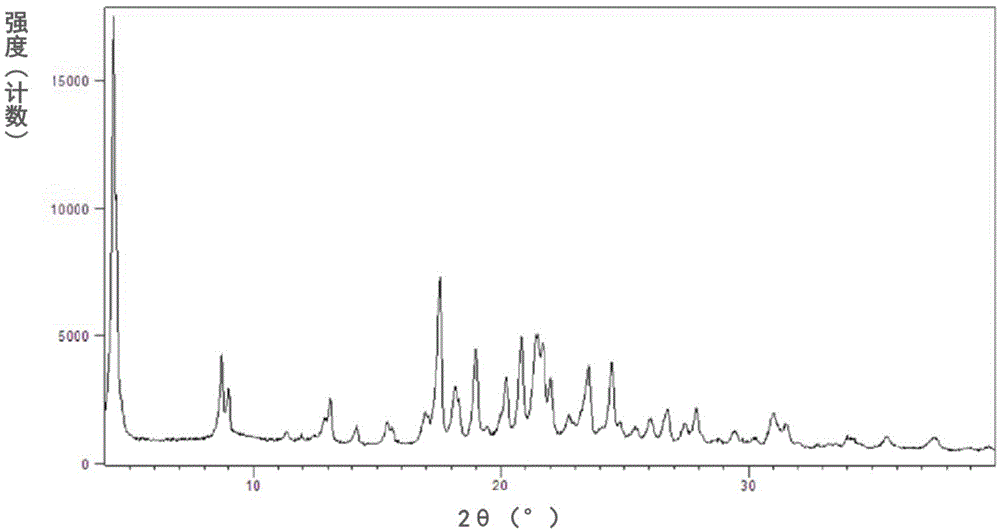
New crystal forms of trelagliptin, and preparation methods and application of crystal forms
InactiveCN105524041AEasy to prepareHigh purityOrganic active ingredientsOrganic chemistryDipeptidyl peptidaseDipeptidyl-Peptidase IV Inhibitors
The invention relates to new crystal forms of trelagliptin, and preparation methods and application of the crystal forms. Specifically, the invention relates to two new crystal forms of trelagliptin as a dipeptidyl peptidase IV inhibitor and preparation methods of the two new crystal forms, a pharmaceutical composition containing the new crystal forms of trelagliptin, and application of the new crystal forms of trelagliptin in manufacturing drugs for treating dipeptidyl-peptidase-IV-mediated diseases.
Owner:SICHUAN HAISCO PHARMA CO LTD
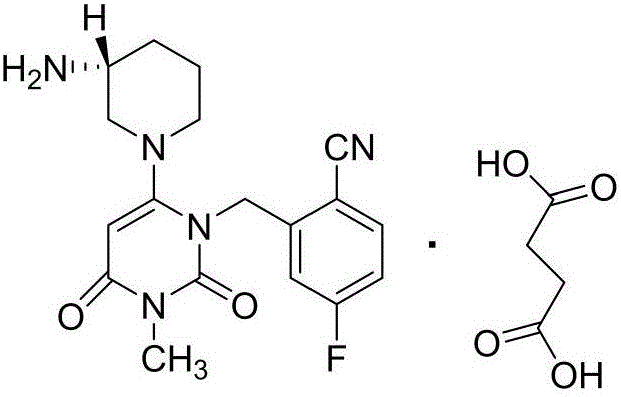
Salt, crystal and pharmaceutical composition of trelagliptin compound and applications thereof
ActiveCN105399725AReduce intakeApplicable to clinical applicationMetabolism disorderOrganic chemistry methodsDiseaseDipeptidyl peptidase
The invention provides a novel dipeptidyl peptidase IV inhibitor trelagliptin compound, hemi-succinate, hydrates and novel crystal-form drugs thereof, a pharmaceutical composition containing them and applications thereof in preparing medications for treating diabetic diseases. A novel crystal form provided by the invention contains 0.5 succinate molecule, so that the intake of inactive ingredients is reduced, then the relative toxicity is low, and the effective composition content is high, therefore, the novel crystal-form is safer and more effective when applied to preparations, and more applicable to the clinical application of high-dose preparations. Compared with the succinate crystal form of existing trelagliptin, the novel crystal form provided by the invention is good in stability and conducive to the wet granulation of solid preparations or the preparation of liquid preparations, and facilitates the manufacturing, storage and transportation of medications; and the novel crystal form provided by the invention is high in purity and safer in usage of medication.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Preparation method of trelagliptin and salt of trelagliptin
ActiveCN108794448AReduce generationSimple separation and purification methodOrganic chemistryAcetic acidPurification methods
The invention discloses a preparation method of trelagliptin and salt of the trelagliptin. The preparation method comprises the following steps: a, stirring a compound 3, Pd(OAc)2, a ligand, K3PO4 and3-tert-butoxycarbonyl-aminopiperidine for a reaction in an organic solvent under the protection of nitrogen to obtain a reaction liquid; b, separating and purifying to obtain a compound 6; c, takingthe compound 6, stirring the the compound 6 with ethyl acetate and an ethyl acetate solution of HCl for the reaction, and separating and purifying to obtain a solid; d, taking the solid of the step c,adding water to dissolve, adjusting the pH to 8-9, and separating and purifying to obtain a compound 4 which is the trelagliptin. By adopting the method provided by the invention, side reactions andthe formation of impurity compounds are reduced; the separation and purification method of trelagliptin is simple, and has the advantages of short production cycle, high yield, high purity, low cost and the like; the yield of trelagliptin of the invention reaches up to 95% or more than 95%; and the preparation method is very suitable for industrial production.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Preparation method for degraded trelagliptin impurity
The invention relates to a preparation method for degraded trelagliptin impurity. The method comprises the following steps: adding 2-[[6-[(3R)-3-amino-1-piperidyl]-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidyl] methyl]-5-fluorobenzonitrile, an oxidizing agent and an acid catalyst into an organic solvent, and then reacting for 6-48h, thereby acquiring the degraded trelagliptin impurity, namely, 2-[[6-[(3R)-3-amino-1-piperidyl]-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidyl] methyl]-5-fluorobenzamide, wherein the organic solvent is selected from at least one of C1-C4 alcohol, C2-C6 ether, tetrahydrofuran and dichloromethane and the oxidizing agent is selected from at least one of hydrogen peroxide, metachloroperbenzoic acid, t-butylhydroperoxide and peracetic acid. The method has the advantages of few steps, simple operation, mild reaction condition, and high product yield and purity.
Owner:GUANGZHOU BAIYUSN GUANGHUA PHARMA
Novel preparation process of trelagliptin succinate
InactiveCN112694465ALow costReduce usageOrganic compound preparationCarboxylic acid salt preparationTrelagliptinSuccinic acid
The invention discloses a novel preparation process of trelagliptin succinate. According to the method, 3-methyl-6-chlorouracil is taken as a starting raw material, toluene, DMF or NMP is taken as a solvent to react with 2-cyano-5-fluorobenzyl bromide under the alkaline condition to obtain 2-[(6-chloro-3, 4-dihydro-3-methyl-2, 4-dioxo-1 (2H)-pyrimidinyl) methyl]-4-fluorobenzonitrile, then the solvent system reacts with (R)-3-Boc-aminopiperidine under the alkaline condition, and the protective group is dissociated by using acid to obtain trelagliptin, and salt forming reaction is carried out on trelagliptin and succinic acid (SM4) to finally obtain the trelagliptin succinate serving as a type 2 diabetes resistant medicine. By adopting a one-pot method, the method has the advantages that the raw material cost is low, the yield is high, the post-treatment operation of each step of chemical reaction in multi-step reaction is reduced, the production period is greatly shortened, few impurities are generated in the reaction, the product quality is high, the use amount of chemical reagents is relatively reduced, and the method is relatively green and environment-friendly, and is beneficial to industrial production.
Owner:山东永丞制药有限公司
Application of asymmetric hydrogenation in synthesis of Trelagliptin intermediate
InactiveCN107445887AMany synthetic stepsThe synthesis method is simpleAsymmetric synthesesState of artTrelagliptin
Owner:SUZHOU XINEN PHARMA
Method for refining trelagliptin
The invention discloses a method for refining trelagliptin, the method comprises the following steps: S1, evenly mixing crude trelagliptin, ethyl acetate and ethanol, refluxing and stirring for 2-4h, adding a molecular sieve, continuing refluxing and stirring for 1-2h, hotly filtering to obtain filtrate, cooling to 0-5 DEG C, stirring for 2-3h, filtering to obtain a filtering cake, and drying to obtain an intermediate; and S2, evenly mixing the intermediate obtained by the step 1 with ethyl acetate and isopropanol, refluxing and stirring for 3-5h, cooling to 0-5 DEG C, stirring for 3-4h, filtering to obtain a filtering cake, and drying to obtain the trelagliptin. The trelagliptin prepared by the method has the advantages of high purity, good yield, simple operation and low cost, and is suitable for industrial production.
Owner:合肥拓锐生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com