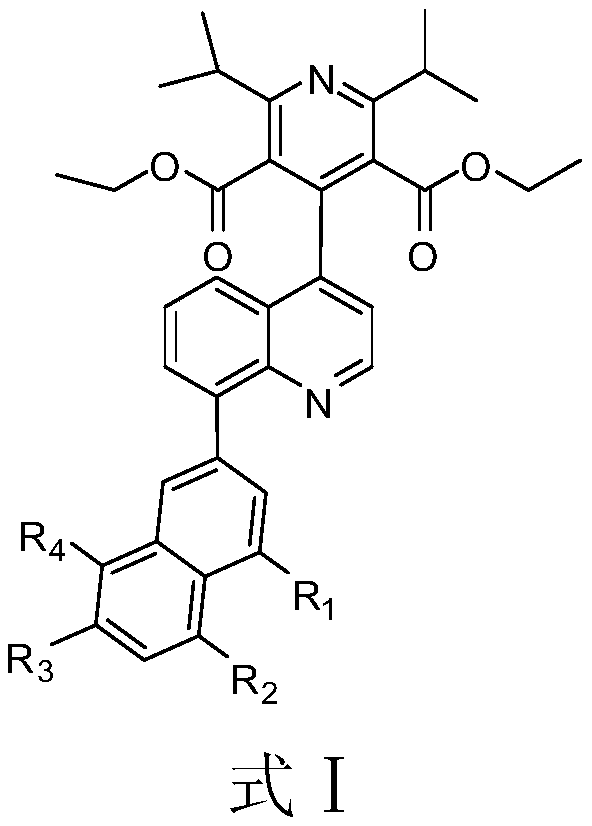
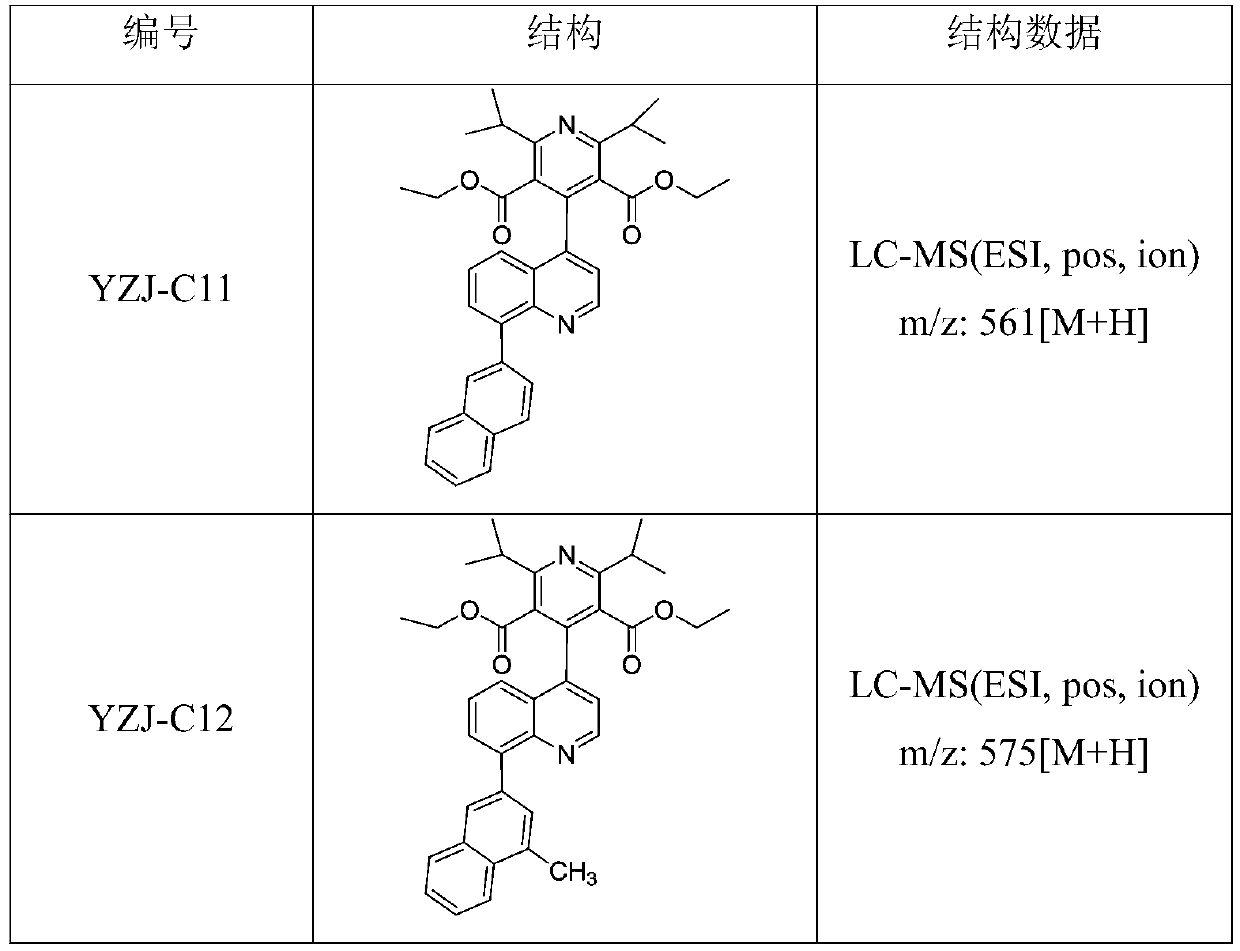
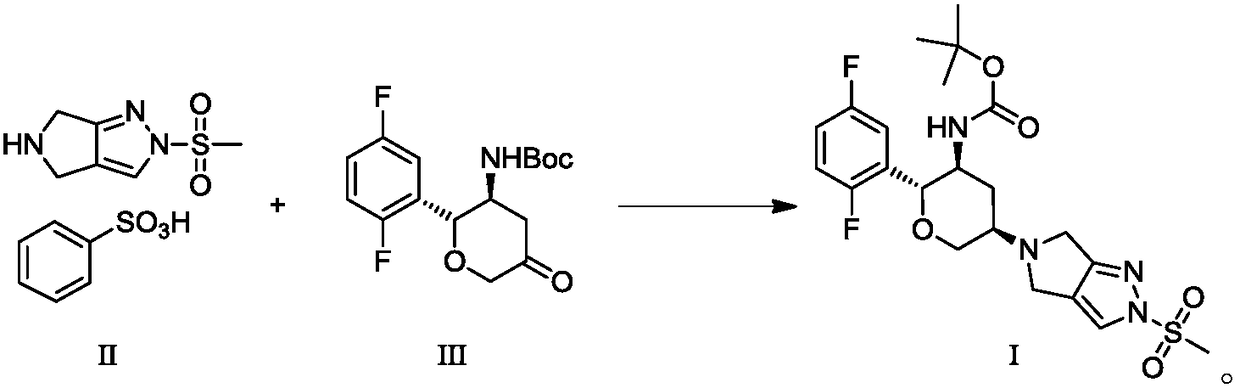
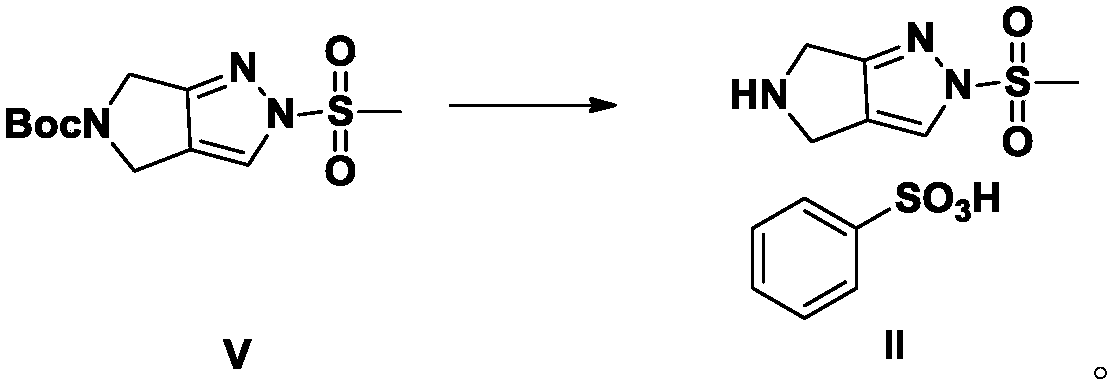
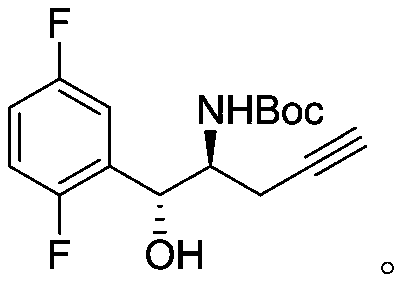
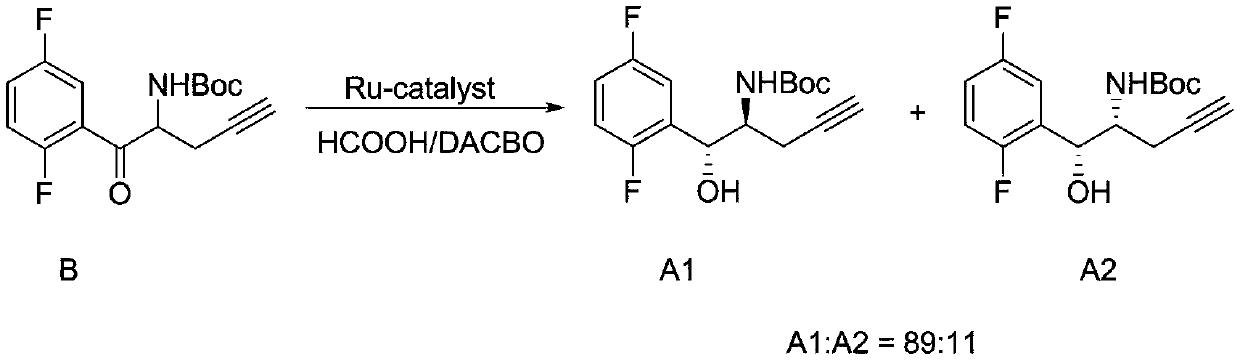
Patents
Literature
43 results about "Omarigliptin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
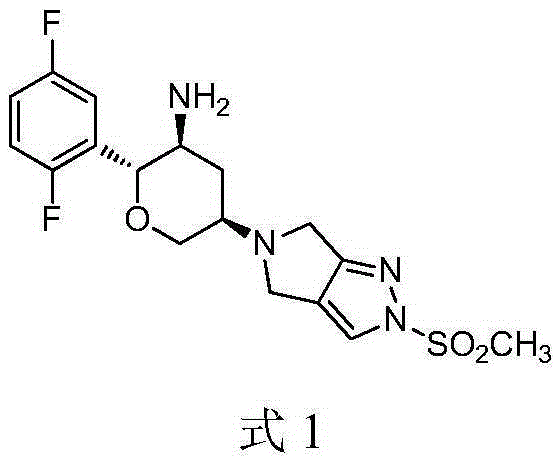
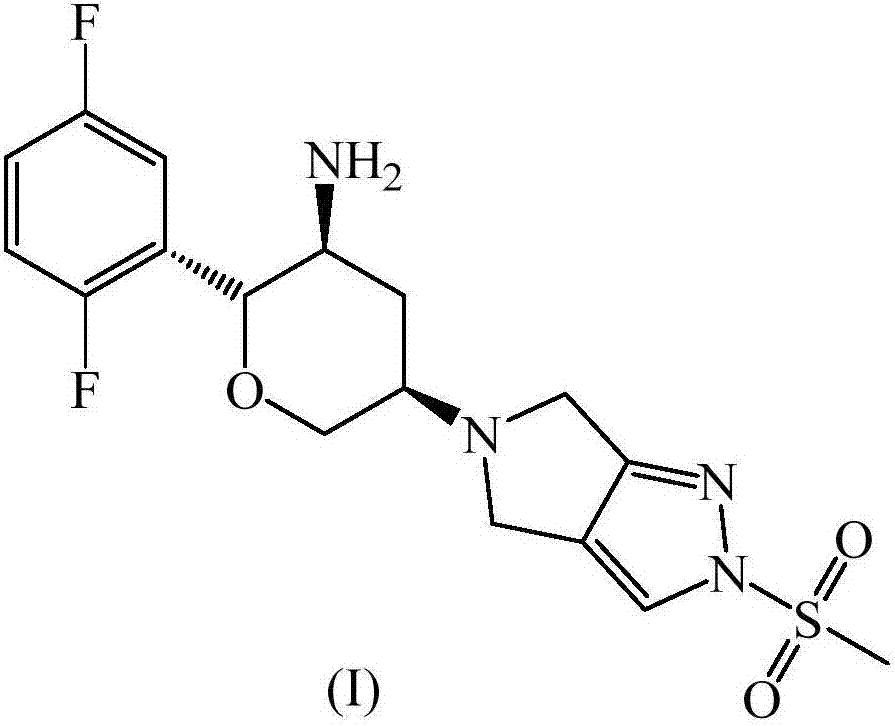
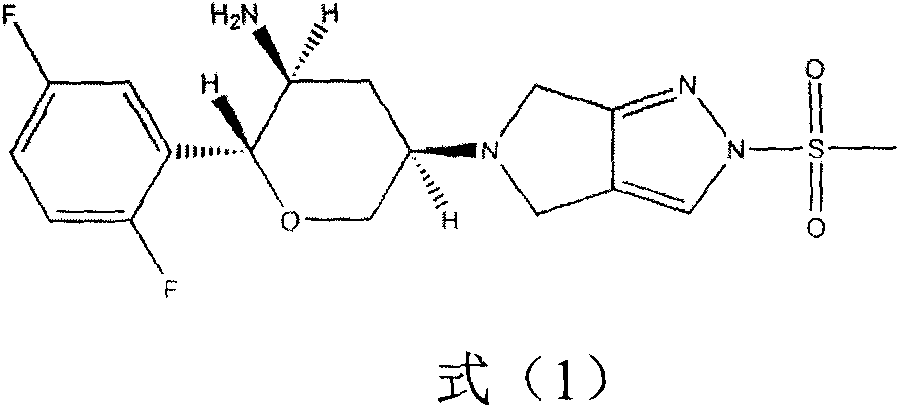
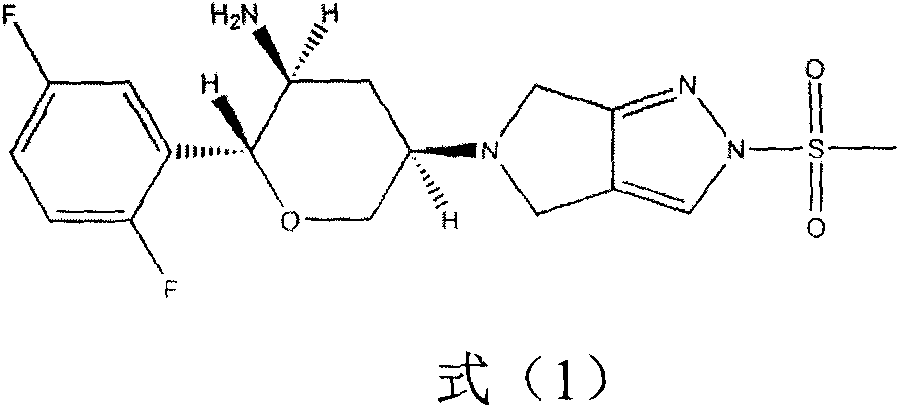
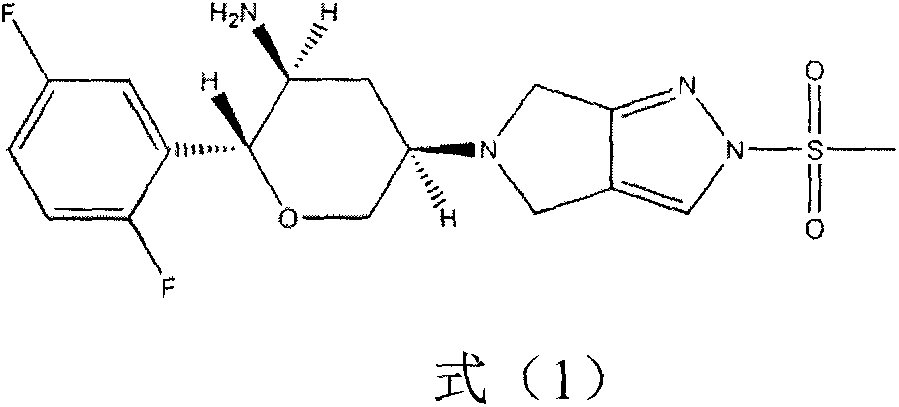
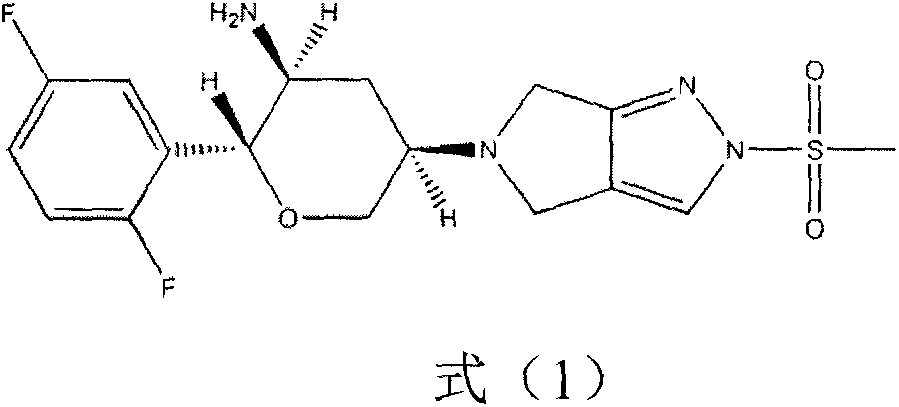
Omarigliptin (MK-3102) is a potent, long-acting oral antidiabetic drug of the DPP-4 inhibitor class used for once-weekly treatment of type 2 diabetes and currently under development by Merck & Co. It inhibits DPP-4 to increase incretin levels (GLP-1 and GIP), which inhibit glucagon release, which in turn increases insulin secretion, decreases gastric emptying and decreases blood glucose levels.
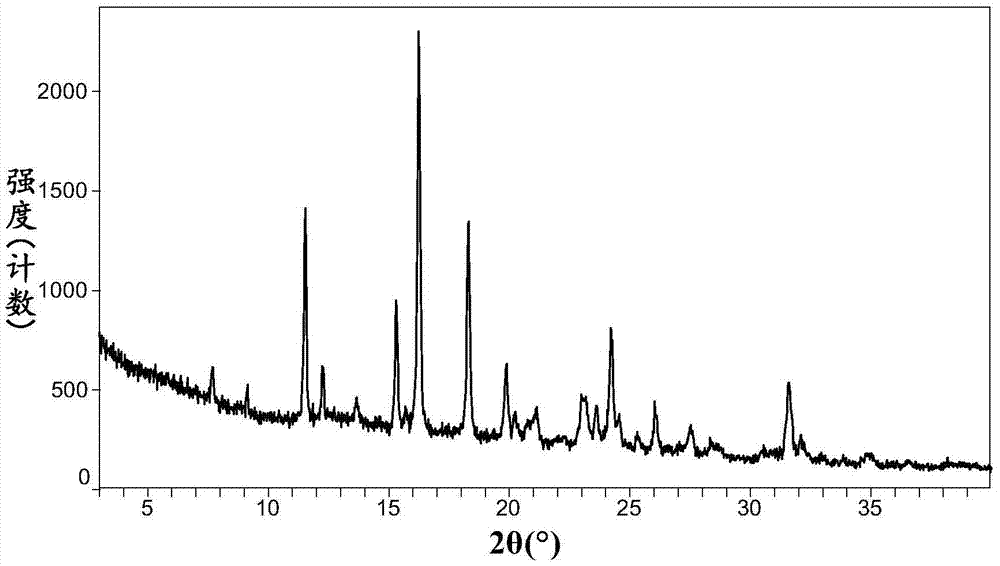
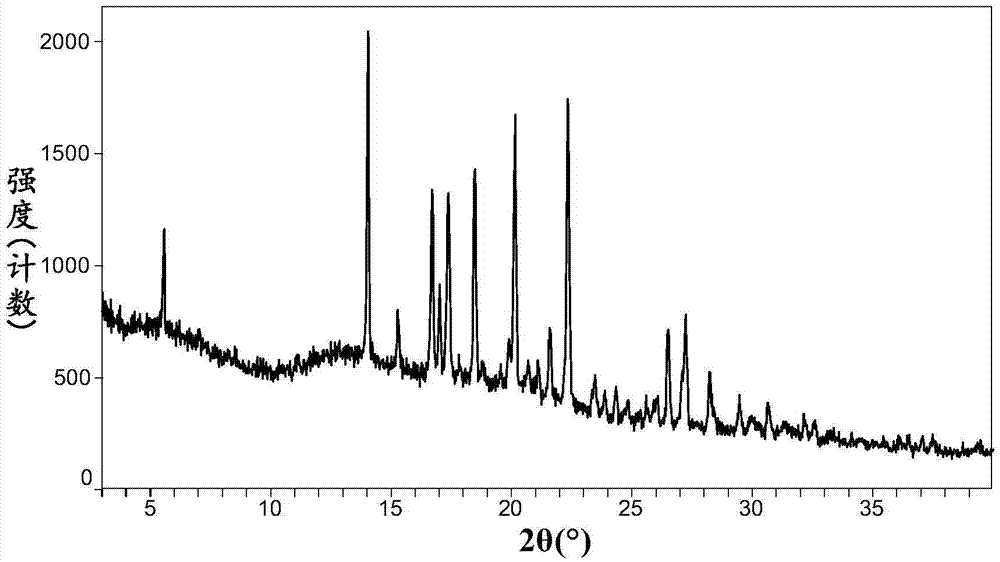
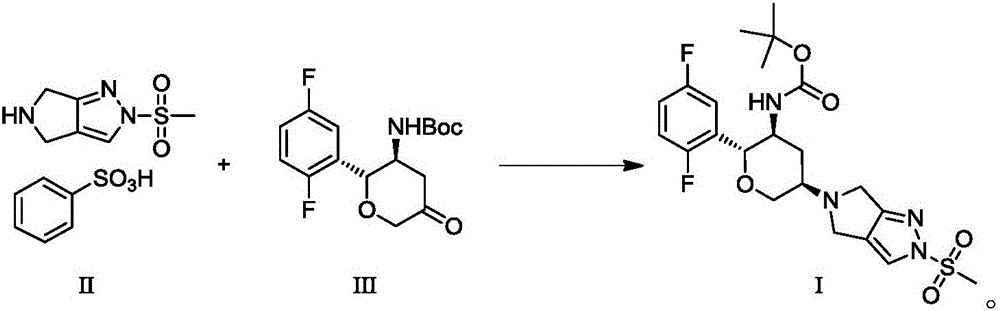
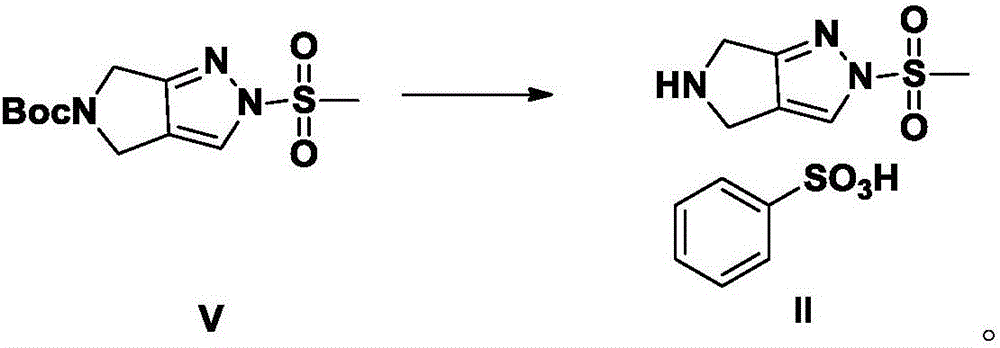
Omarigliptin salt, crystal form products of omarigliptin salt, preparing methods of omarigliptin salt and crystal form products and pharmaceutical composition
ActiveCN106928228AImprove solubilityFast dissolutionOrganic active ingredientsMetabolism disorderPharmaceutical drugBioavailability
The invention relates to novel omarigliptin salt and crystal form products of the omarigliptin salt, and belongs to the technical field of pharmaceutical chemicrystallization. Compared with existing omarigliptin crystal form products, the omarigliptin salt and the crystal form products of the omarigliptin salt have the advantages of being better in crystal form product stability and solubility, higher in dissolving rate and the like, the bioavailability of medicine can be improved, and the omarigliptin salt and the crystal form products of the omarigliptin salt are better suitable for an application of a solid preparation. The invention also relates to the omarigliptin salt, a preparing method of the crystal form products of the omarigliptin salt, a pharmaceutical composition of the omarigliptin salt and applications of the omarigliptin salt to medicine for treating type 2 diabetes mellitus.
Owner:SOLIPHARMA
Synthetic method for omarigliptin
InactiveCN105399744AMany synthetic stepsThe synthesis method is simpleOrganic chemistryRaw materialOmarigliptin
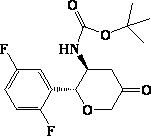
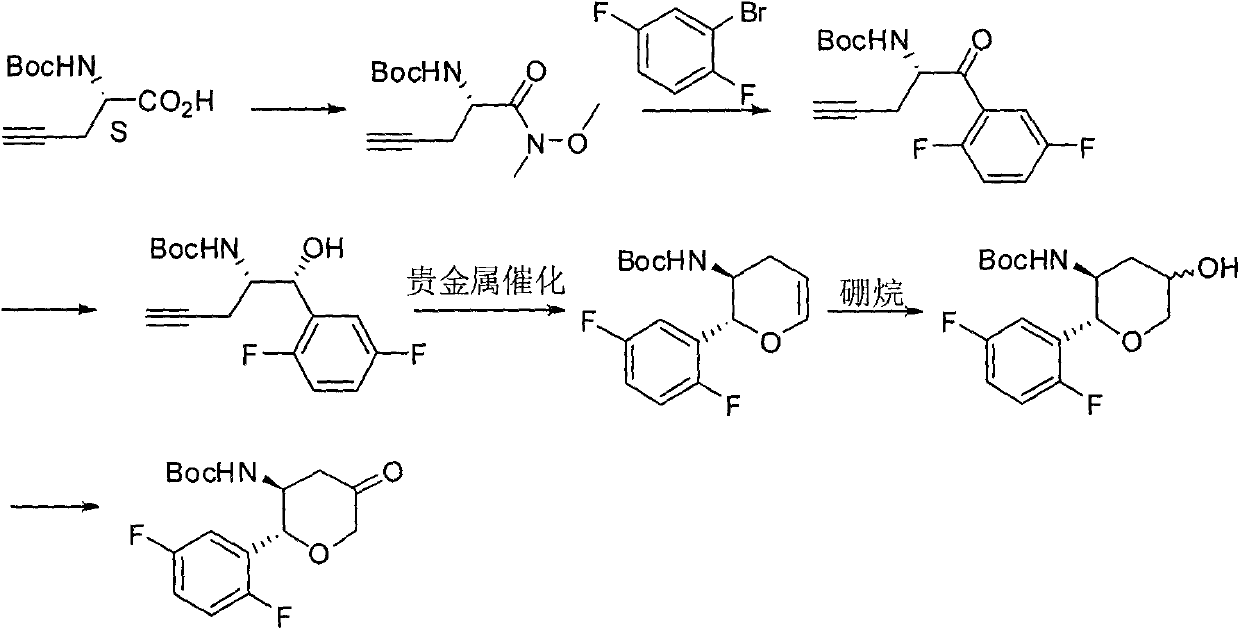
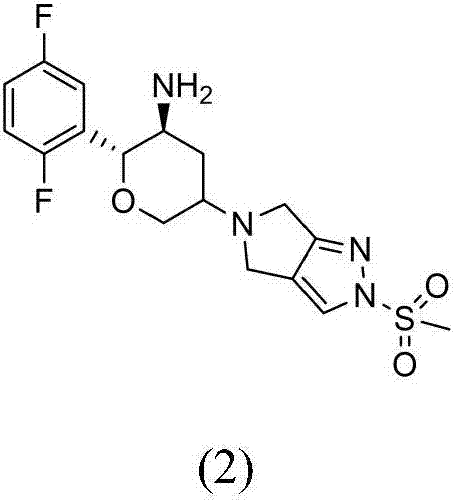
The invention provides a synthetic method for a formula (I) compound omarigliptin. A formula (2) compound and a formula (3) compound are taken as initial raw materials and are subjected to a series of reactions shown in the specification, so that the formula (1) compound omarigliptin is finally prepared. Compared with the prior art defects that omarigliptin synthetic steps are more and synthetic technology is complex, the synthetic method is simple and practicable, relatively low in cost, relatively high in yield, relatively good in product quality, and is suitable for large-batch industrialized production.
Owner:黄燕鸽
Combination product containing limonin compound and DPP-4 inhibitors
The invention relates to a combination product of a limonin compound, pharmaceutically-acceptable derivatives, esters, stereoisomers, salts or prodrugs thereof and dipeptidyl peptidase-4(DPP-4) inhibitors such as sitagliptin, saxagliptin, vildagliptin, linagliptin, alogliptin, trelagliptin and omarigliptin. The invention further relates to application of the combination product in treating and / orpreventing diseases related to diabetes.
Owner:NATURAL MEDICINE INST OF ZHEJIANG YANGSHENGTANG
Omarigliptin intermediate preparation method
The invention provides an omarigliptin intermediate preparation method. The omarigliptin intermediate preparation method comprises the step of mixing a compound I with a catalyzing enzyme, and reacting to obtain an omarigliptin intermediate. The omarigliptin intermediate preparation method has the beneficial effects that the cost of raw materials is reduced; the problem in the prior art that isomer byproducts are generated during preparation of the omarigliptin intermediate and cannot be utilized is effectively solved; the yield and atom economy are increased; and the omarigliptin intermediate preparation method is simple in operation, mild in conditions, environment-friendly and suitable for industrial production.
Owner:SUZHOU LEAD BIOTECH CO LTD
Preparation method of omarigliptin intermediate
ActiveCN107325020ALow priceEliminate long synthetic stepsCarbamic acid derivatives preparationOrganic compound preparationState of artCarbamate
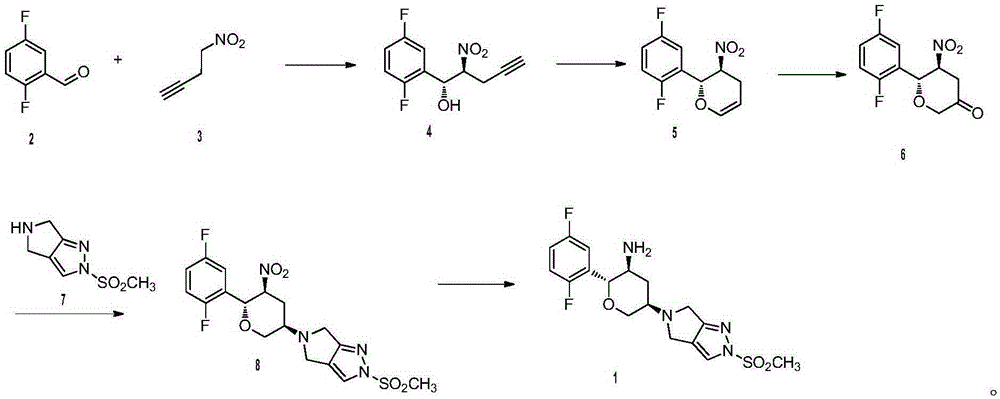
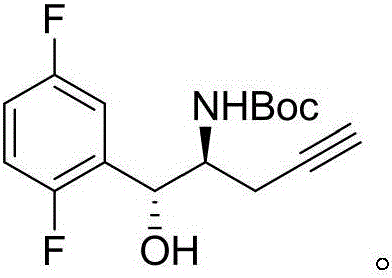
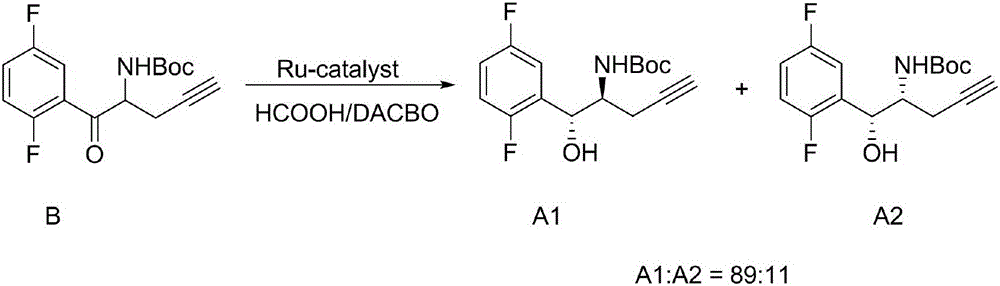
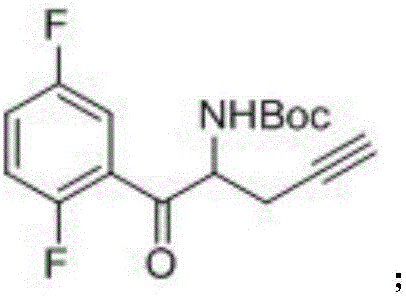
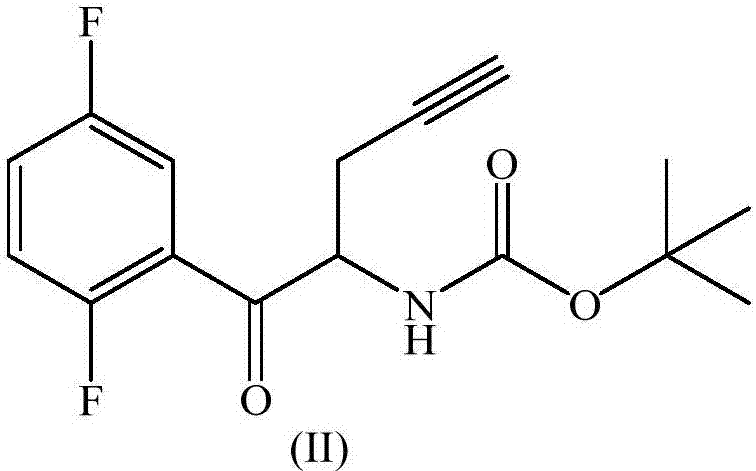
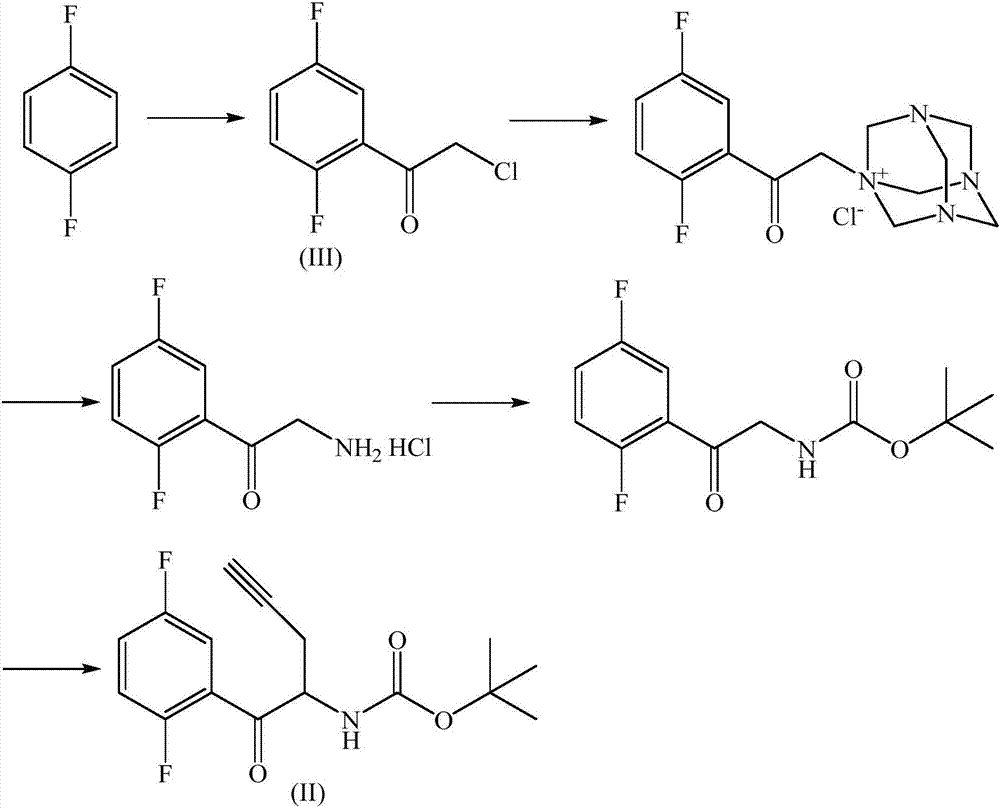
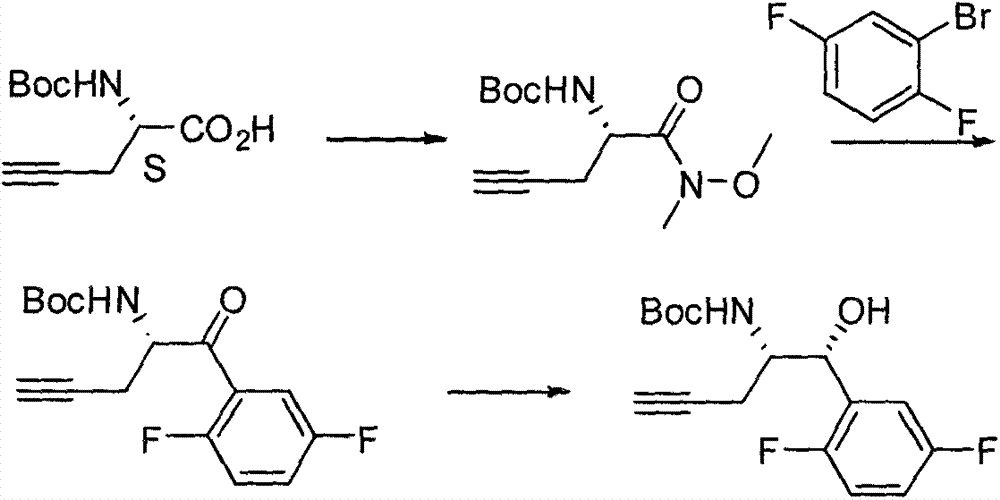
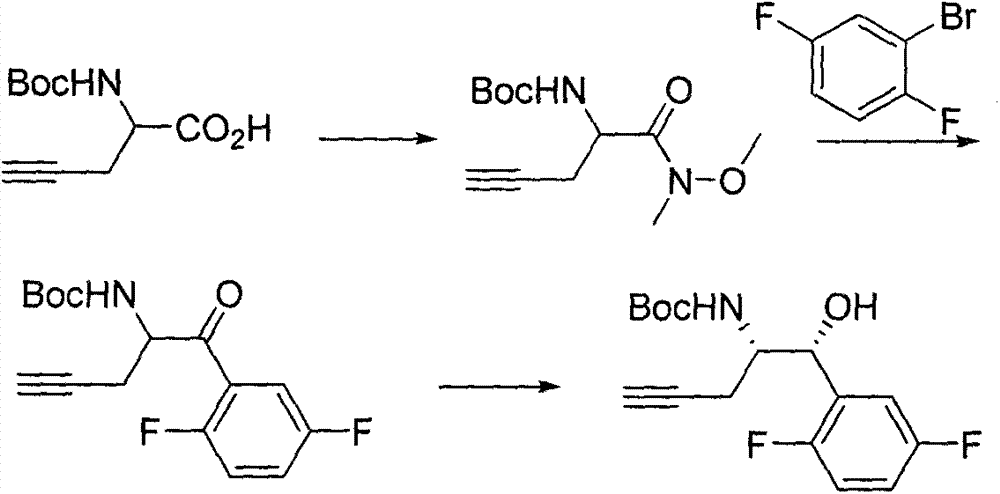
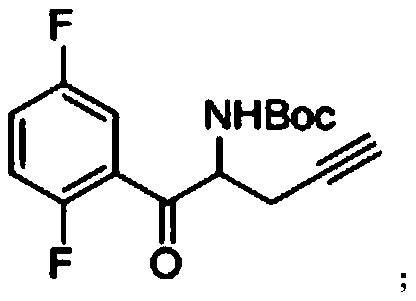
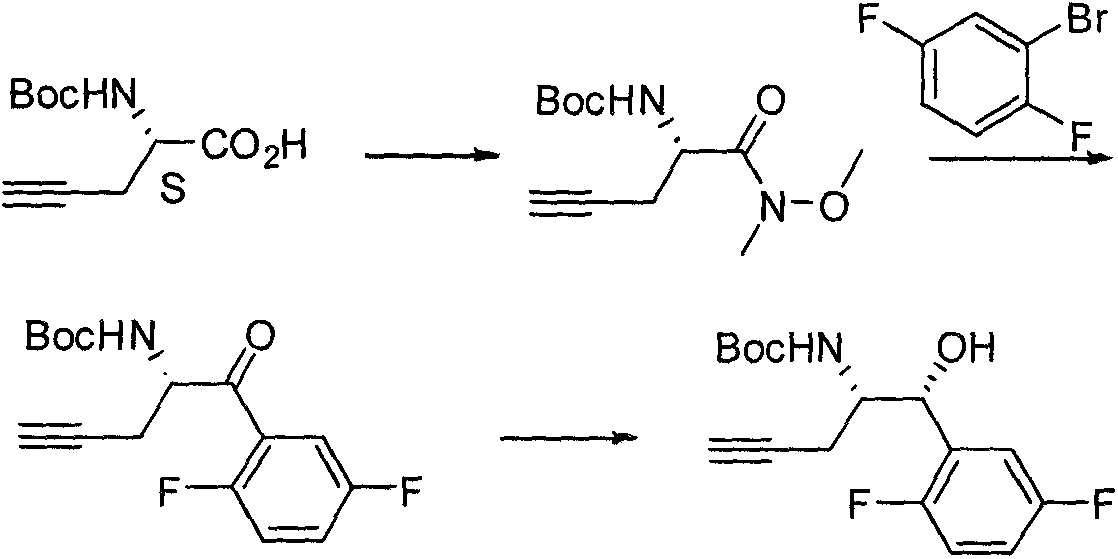
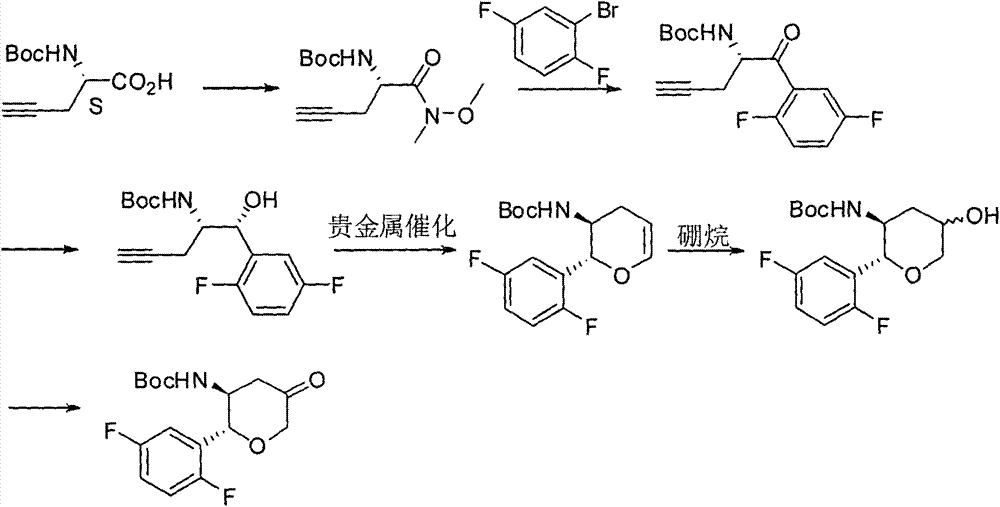
The invention relates to a preparation method of an omarigliptin intermediate. The omarigliptin intermediate is tert-butyl [1-(2,5-difluorophenyl)-1-oxopent-4-yn-2-yl] carbamate. The method comprises the following steps: a compound I shown as the formula (III) is prepared from 1,4-difluorobenzene as a starting material; the compound I is subjected to a reaction with dibenzimide, and a compound II shown as the formula (IV) is obtained; the compound II is subjected to a substitution reaction with a propargyl compound, and a compound III shown as the formula (V) is prepared; the compound III is subjected to acidolysis and Boc protection, and the compound, namely, the omarigliptin intermediate, shown as the formula (II) is obtained. According to the preparation method, technical defects of long synthesis steps, quite expensive starting materials such as 2,5-difluorobenzaldehyde or 2-bromo-1,4-difluorobenzene, preparation of Weinreb amides, use of CDI (1,1'-Carbonyldiimidazole) and the like in the prior art are overcome; the preparation method has the advantages of simple operation, high yield, low cost and wide source of raw materials and the like, and is applicable to industrial production.
Owner:GUANGXI UNIVERSITY OF TECHNOLOGY
Omarigliptin and preparation method of intermediate of Omarigliptin
ActiveCN106674227AEasy to operateSimple and safe operationOrganic chemistry methodsBulk chemical productionOrganic solventSodium borohydride
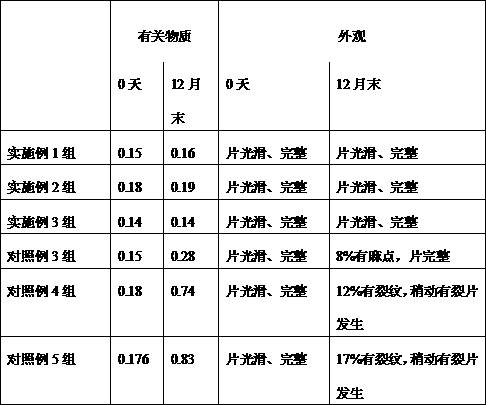
The invention discloses Omarigliptin and a preparation method of an intermediate of the Omarigliptin, and provides a preparation method of an Omarigliptin intermediate I. The preparation method comprises the following step: enabling a compound II and a compound III to undergo a condensation reaction in a polar aprotic organic solvent in the presence of lewis acid and sodium borohydride acetate to obtain the Omarigliptin intermediate I. The preparation method disclosed by the invention has the advantages of simple and safe operation, no need of special purification equipment, short reaction time, fewer by products, high yield and simple post treatment operation; column chromatography separating operation in a post treatment process is avoided; a prepared product is high in purity, the optical purity is greater than or equal to 99,9 percent, the purity of a related substance is greater than or equal to 98.5 percent, and the purities of all impurities are smaller than or equal to 0.5 percent separately; the preparation method is low in production cost and is suitable for industrial production. In addition, according to the Omarigliptin prepared from the Omarigliptin intermediate I obtained by adopting the preparation method disclosed by the invention, the purity is greater than or equal to 99.5 percent, the purities of all the impurities are smaller than or equal to 0.1 percent separately, and the standards of crude drugs are reached. (The formula is shown in the description).
Owner:上海云晟研新生物科技有限公司
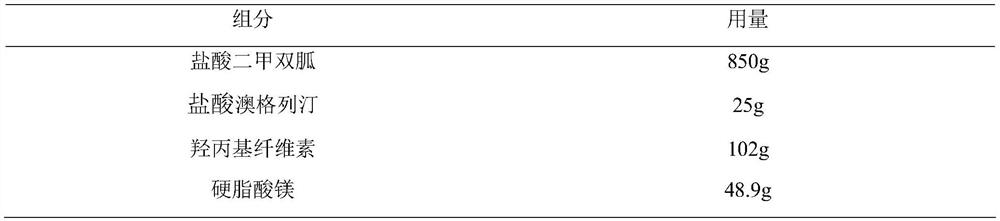
Pharmaceutical composition taking metformin or pharmaceutical salts thereof and omarigliptin or pharmaceutical salts thereof as active ingredients
InactiveCN112641776AUnsatisfactory treatmentUnsatisfactory treatment effectOrganic active ingredientsMetabolism disorderMetformin hclBULK ACTIVE INGREDIENT
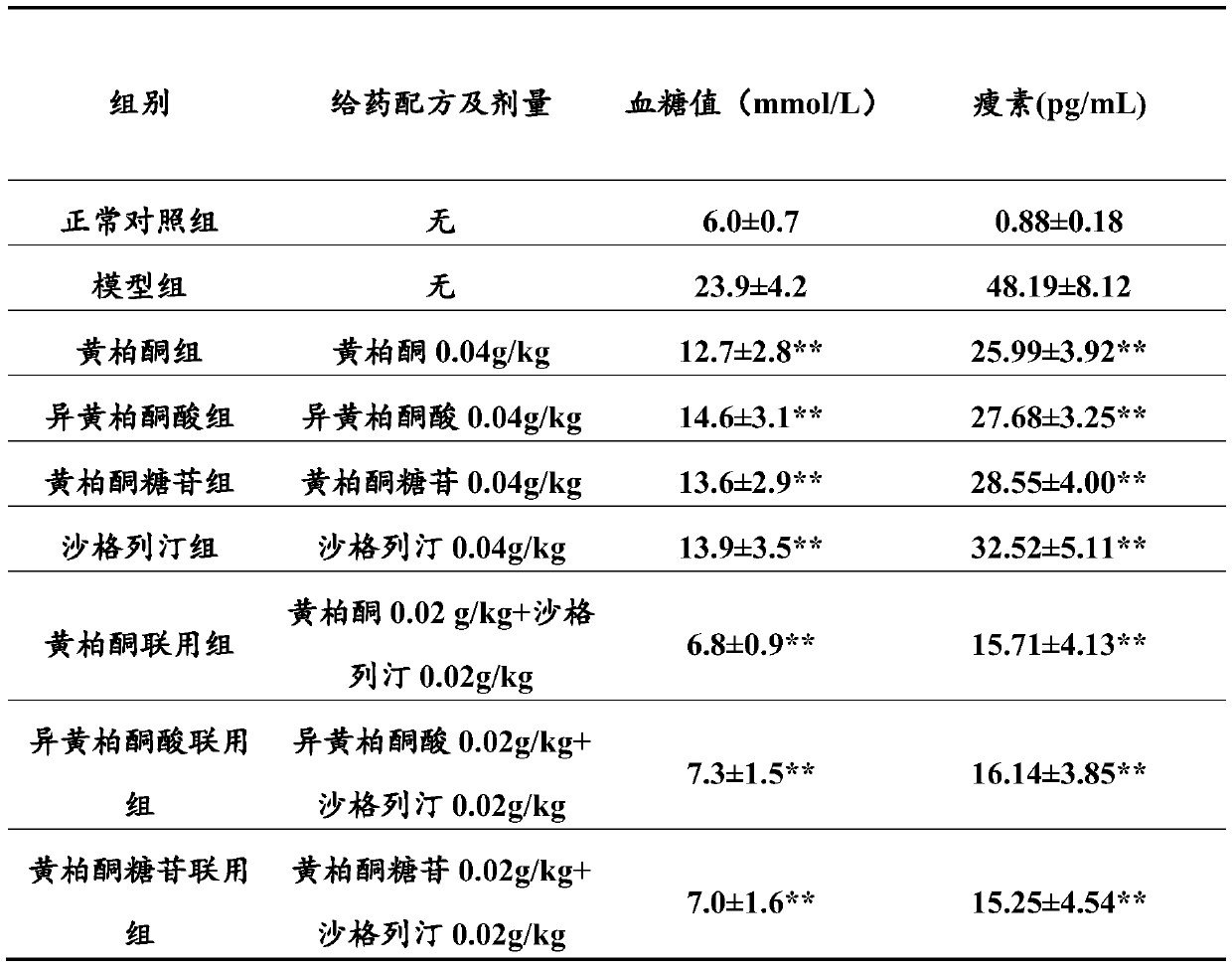
The invention discloses a pharmaceutical composition taking metformin or pharmaceutical salts thereof and omarigliptin or pharmaceutical salts thereof as active ingredients. Pharmacological research results show that the pharmaceutical composition of metformin hydrochloride and omarigliptin hydrochloride can improve the pancreatic islet form of a test animal, effectively reduce the blood glucose level of a diabetic model animal and improve glucose tolerance; the hypoglycemic effect is dose-dependent; and furthermore, the hypoglycemic effect is rapid, stable and lasting.
Owner:JIANGSU JINGLIXIN PHARMA TECH CO LTD
DPP-4 inhibitor, preparation method thereof and application of DPP-4 inhibitor in diabetes mellitus
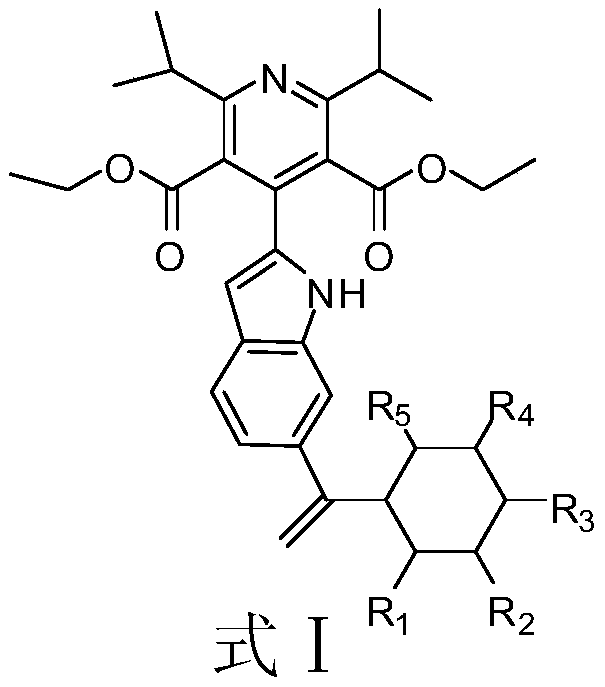
The invention discloses a compound or pharmaceutically acceptable salt thereof shown as a formula I (the formula l is shown in the following description), wherein R1, R2 and R3 are each independentlyselected from H or OCH3. In an in-vitro DDP-4 enzyme inhibition test, IC50 values of listed compounds to DDP-4 are all smaller than omarigliptin and Sitagliptin. The test shows that the compound provided by the invention has better DDP-4 inhibition activity and can be used for deeper study as a drug for treating and / or preventing non-insulin-dependent diabetes mellitus, hyperglycemia or insulin resistance.
Owner:中昱医学检验(广州)有限公司
Omarigliptin tablet composition
InactiveCN107802607ASolve hygroscopicitySolve the problem of elevated substancesOrganic active ingredientsMetabolism disorderCarboxymethyl celluloseHydrogen phosphate
The invention relates to an alogliptin composition and belongs to the technical field of pharmacy. The technical scheme of the present invention is: in the alogliptin composition of unit dosage, contain the alogliptin 12.5-25 mg that passes 100 mesh sieves, the chitosan 12-20mg that molecular weight is 1.8-20,000, calcium hydrogen phosphate 16‑32mg, corn starch 16‑30mg, microcrystalline cellulose 20‑36mg, lactose 20‑40mg, croscarmellose sodium 8‑15mg, sodium lauryl sulfate 1.2‑1.6mg, magnesium stearate 0.8-1.5 mg. The invention provides an alogliptin tablet composition with stable quality.
Owner:WEIHAI GUANBIAO INFORMATION TECH
Synthesizing method of omarigliptin
InactiveCN107235983AMany synthetic stepsThe synthesis method is simpleOrganic chemistryState of artPhotochemistry
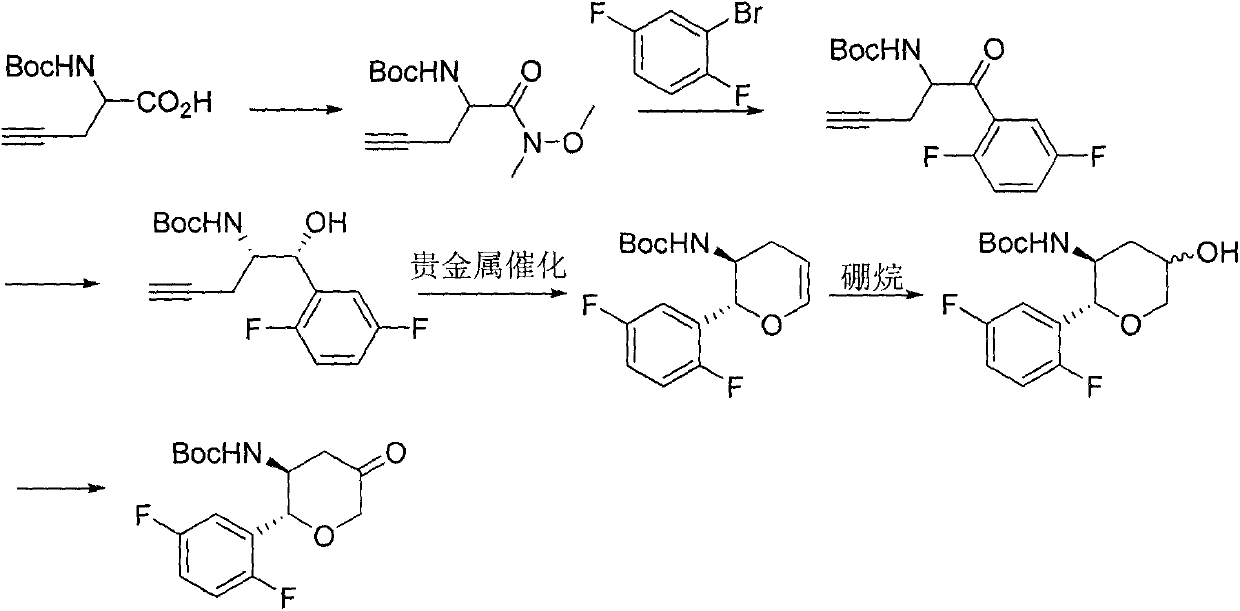
The invention provides a synthesizing method of an omarigliptin compound shown in a formula (1). The synthesizing method comprises the following steps: by using a compound shown in a formula (2) as an initial raw material, performing a series of following reaction, so as to finally obtain the compound shown in the formula (1), namely omarigliptin, wherein reaction formulae are shown in the description. Compared with the prior art with multiple synthesizing steps and complicated synthesizing technology for synthesizing the omarigliptin, the synthesizing method has the advantages that the method is simple, the implementing is easy, the cost is lower, the yield rate is higher, the product quality is higher, and the synthesizing method is suitable for large-scale industrialized production.
Owner:SUZHOU XINEN PHARMA
Process for the preparation of key intermediates of omarigliptin
ActiveUS9481680B2Carbamic acid derivatives preparationOrganic compound preparationCombinatorial chemistryBULK ACTIVE INGREDIENT
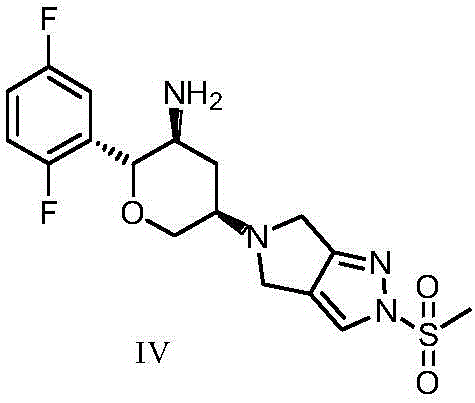
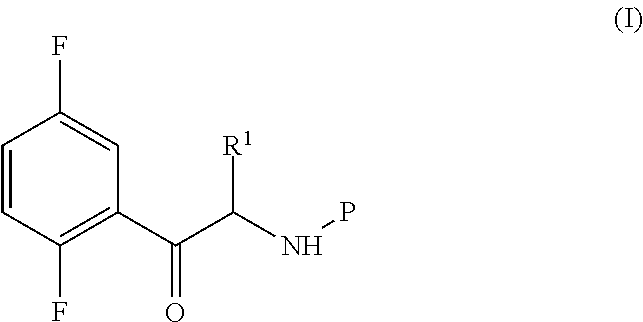
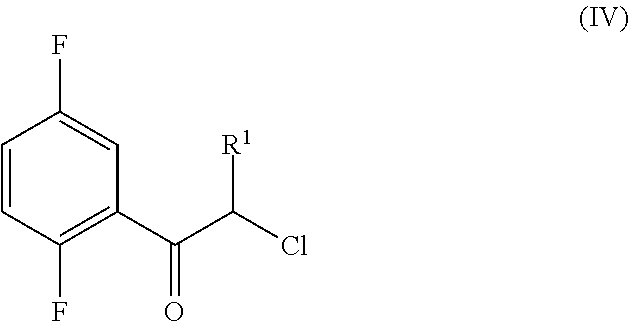
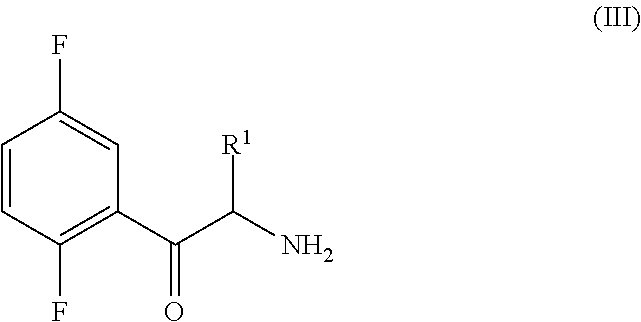
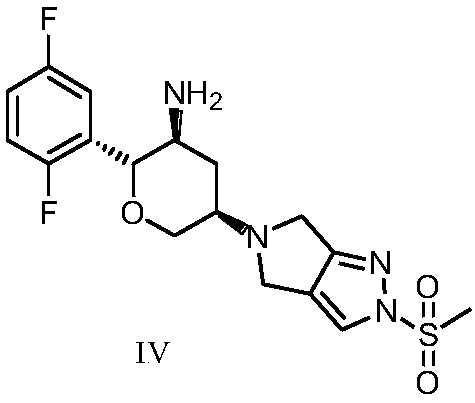
An improved process for the preparation of a key intermediate for the synthesis of the active ingredient Omarigliptin is provided. The key intermediate is a compound having the following formula (I)wherein R1 is propargyl or allyl group and P is an amine protecting group. The compound of formula (I) is prepared by converting a compound of formula (IV)by an amination reaction to a compound of formula (III),which is then protected to provide a compound of formula (II),which is then alkylated to provide the compound of formula (I).
Owner:F I S FAB ILTALIANA SINTETICI SPA
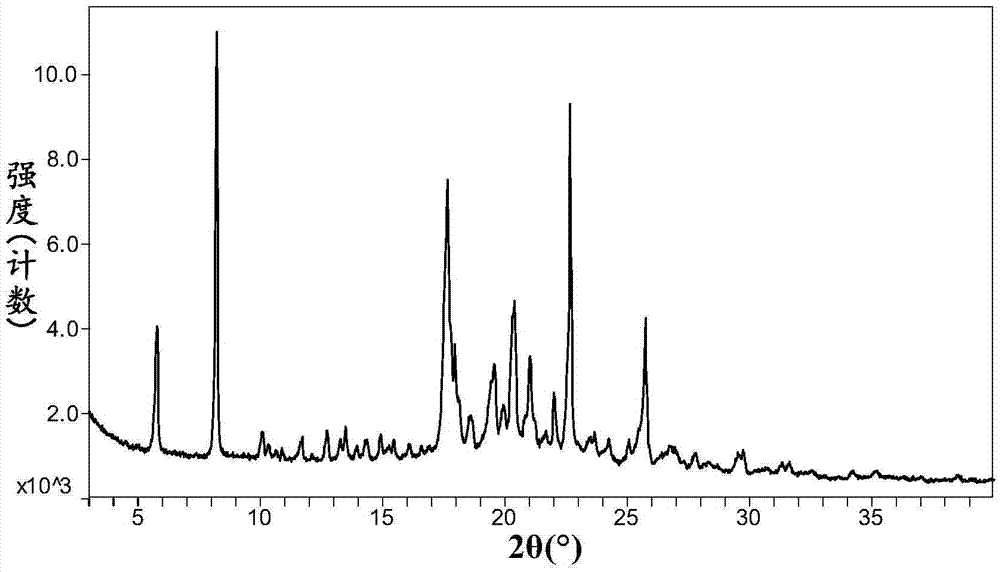
Alogliptin salt and its crystal form, their preparation method and pharmaceutical composition
ActiveCN106928228BImprove solubilityFast dissolutionOrganic active ingredientsOrganic compound preparationSolubilityAlogliptin
The invention relates to novel omarigliptin salt and crystal form products of the omarigliptin salt, and belongs to the technical field of pharmaceutical chemicrystallization. Compared with existing omarigliptin crystal form products, the omarigliptin salt and the crystal form products of the omarigliptin salt have the advantages of being better in crystal form product stability and solubility, higher in dissolving rate and the like, the bioavailability of medicine can be improved, and the omarigliptin salt and the crystal form products of the omarigliptin salt are better suitable for an application of a solid preparation. The invention also relates to the omarigliptin salt, a preparing method of the crystal form products of the omarigliptin salt, a pharmaceutical composition of the omarigliptin salt and applications of the omarigliptin salt to medicine for treating type 2 diabetes mellitus.
Owner:SOLIPHARMA
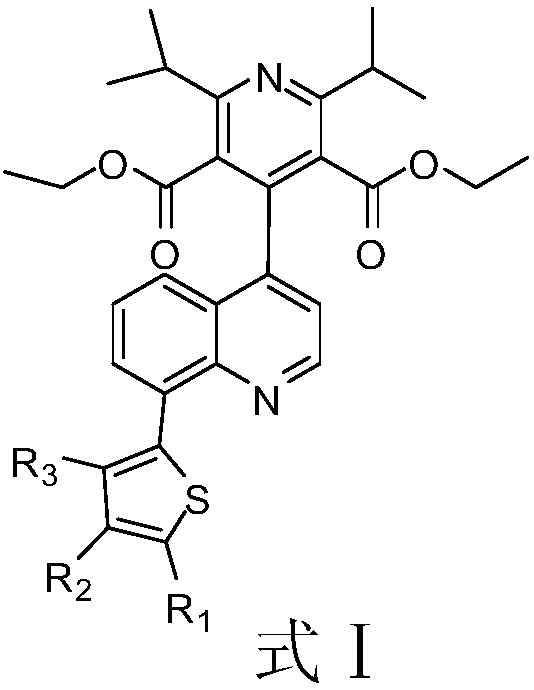
A kind of quinoline derivative and its application in diabetes
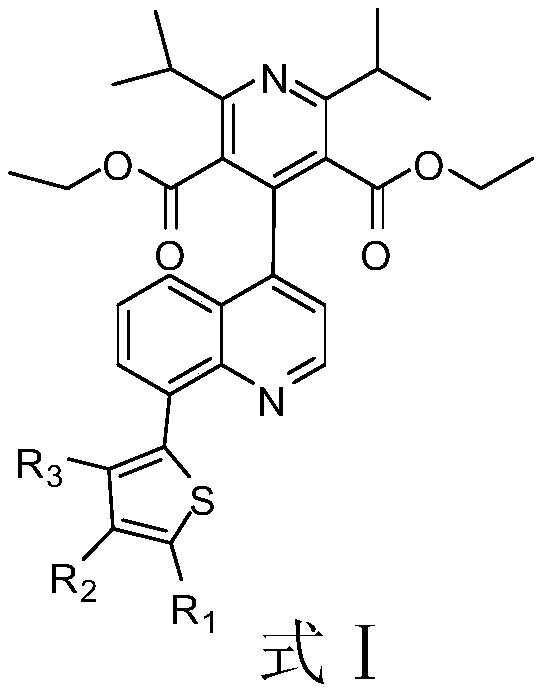
The invention discloses a quinoline derivative or pharmacologically acceptable salt shown as a formula I (The formula is shown in the description), wherein R1, R2, R3 and R4 are respectively selectedfrom H or CH3. The compound listed in an in vitro DPP-4 enzyme inhibition test on the inhibition activity of DPP-4 is between Sitagliptin and omarigliptin. The quinoline derivative shown as the formula I disclosed by the invention is a DPP-4 inhibitor with a novel structure type, and the characteristics of the quinoline derivative in pharmacological toxicology and pharmacokinetics deserve more in-depth study, so that drugs used for treating and / or preventing non-insulin-dependent diabetes, hyperglycemia or insulin resistance can be obtained.
Owner:TONGCHUAN PEOPLES HOSPITAL
DP-IV inhibitor drug composition
PendingCN109771651AImprove stabilityHigh API contentOrganic active ingredientsMetabolism disorderDigestionOmarigliptin
The invention provides a DP-IV inhibitor drug composition which comprises Omarigliptin and reducing sugar. According to the DP-IV inhibitor drug composition, the prejudice is overcome, an Omarigliptindomestic preparation is provided, the cost is low, the digestion is consistent with that of the original drug, and the stability is higher than that of the original drug.
Owner:HENAN MEDICAL COLLEGE
Preparation method of omarigliptin intermediate
ActiveCN107473988AStable in natureRaw materials are easy to getCarbamic acid derivatives preparationOrganic compound preparationAlderSynthesis methods
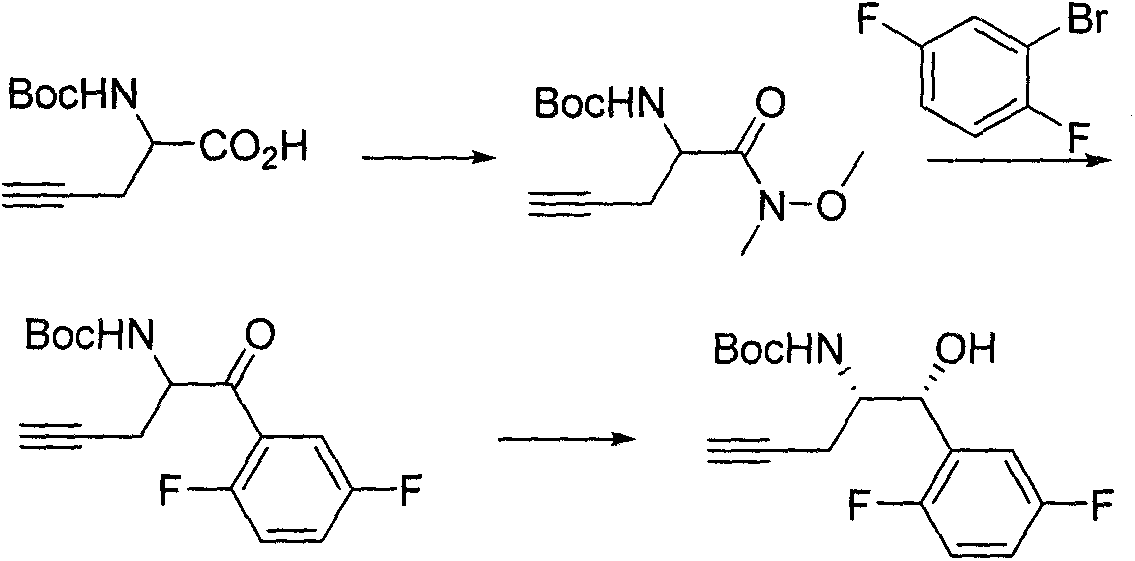
The invention discloses a novel synthesis method for preparing a chiral intermediate of omarigliptin. The method disclosed by the invention comprises the following steps: taking crotonyl chloride and (S)-4-benzyl-2-oxazolidone as starting raw materials and carrying out amidation, alder condensation, hydrolysis, Curtius rearrangement, Boc addition and ring opening to obtain the chiral intermediate (VI). The preparation method disclosed by the invention has the advantages of relatively short route, relatively low cost, easiness for obtaining the raw materials and relatively high yield so that the preparation method is suitable for industrial production. A formula is shown in the description.
Owner:ANHUI HAIKANG PHARMA
A kind of preparation method of alogliptin and its intermediate
ActiveCN106674227BEasy to operateSimple and safe operationOrganic chemistryBulk chemical productionImpurityBy-product
The invention discloses Omarigliptin and a preparation method of an intermediate of the Omarigliptin, and provides a preparation method of an Omarigliptin intermediate I. The preparation method comprises the following step: enabling a compound II and a compound III to undergo a condensation reaction in a polar aprotic organic solvent in the presence of lewis acid and sodium borohydride acetate to obtain the Omarigliptin intermediate I. The preparation method disclosed by the invention has the advantages of simple and safe operation, no need of special purification equipment, short reaction time, fewer by products, high yield and simple post treatment operation; column chromatography separating operation in a post treatment process is avoided; a prepared product is high in purity, the optical purity is greater than or equal to 99,9 percent, the purity of a related substance is greater than or equal to 98.5 percent, and the purities of all impurities are smaller than or equal to 0.5 percent separately; the preparation method is low in production cost and is suitable for industrial production. In addition, according to the Omarigliptin prepared from the Omarigliptin intermediate I obtained by adopting the preparation method disclosed by the invention, the purity is greater than or equal to 99.5 percent, the purities of all the impurities are smaller than or equal to 0.1 percent separately, and the standards of crude drugs are reached. (The formula is shown in the description).
Owner:SHANGHAI BOCIMED PHARMA CO LTD
A kind of method for preparing alogliptin intermediate
The invention provides an omarigliptin intermediate preparation method. The omarigliptin intermediate preparation method comprises the step of mixing a compound I with a catalyzing enzyme, and reacting to obtain an omarigliptin intermediate. The omarigliptin intermediate preparation method has the beneficial effects that the cost of raw materials is reduced; the problem in the prior art that isomer byproducts are generated during preparation of the omarigliptin intermediate and cannot be utilized is effectively solved; the yield and atom economy are increased; and the omarigliptin intermediate preparation method is simple in operation, mild in conditions, environment-friendly and suitable for industrial production.
Owner:SUZHOU LEAD BIOTECH CO LTD
A kind of quinoline derivative and its application in diabetes
Owner:DEZHOU UNIV
The preparation method of the intermediate of alogliptin
ActiveCN107473988BStable in natureRaw materials are easy to getCarbamic acid derivatives preparationOrganic compound preparationAlogliptinSynthesis methods
The invention discloses a novel synthesis method for preparing a chiral intermediate of omarigliptin. The method disclosed by the invention comprises the following steps: taking crotonyl chloride and (S)-4-benzyl-2-oxazolidone as starting raw materials and carrying out amidation, alder condensation, hydrolysis, Curtius rearrangement, Boc addition and ring opening to obtain the chiral intermediate (VI). The preparation method disclosed by the invention has the advantages of relatively short route, relatively low cost, easiness for obtaining the raw materials and relatively high yield so that the preparation method is suitable for industrial production. A formula is shown in the description.
Owner:ANHUI HAIKANG PHARMA
A kind of dpp-4 inhibitor and its preparation and application in diabetes
The invention discloses a compound shown in a formula I or pharmaceutically acceptable salts of the compound; the formula I is described in the description, wherein R1, R2, R3, R4 and R5 are independently selected from H or CF3. The IC50 values, for the dipeptidyl peptidase-IV (DPP-4), of compounds listed in in-vitro DPP-4 enzyme inhibition tests are less than those of omarigliptin and Sitagliptin. The compound provided by the invention is proved to have better DPP-4 inhibitory activity, thus being used as a medicine for treating and / or preventing non-insulin-dependent diabetes, hyperglycemiaor insulin resistance for further study.
Owner:张爱芳
New method for preparing omarigliptin key intermediate
The invention provides a new method for preparing an omarigliptin key intermediate. The method comprises the following steps that 2,5-difluorobenzaldehyde is used as a raw material, and through a 7-step reaction, the omarigliptin key intermediate is obtained. According to the method for preparing the omarigliptin key intermediate disclosed by the invention, a new synthesis route for preparing theomarigliptin key intermediate is provided, the cost of raw materials and the cost of reagents are reduced, the yield of the omarigliptin intermediate is increased, the reaction operation is simple, the condition is mild, and the method is environmentally friendly and suitable for industrial production.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
A kind of preparation method of the chiral intermediate of alogliptin
ActiveCN107459501BStable in natureRaw materials are easy to getOrganic chemistry methodsPtru catalystOxazolidone
The invention discloses a novel synthesis method for preparing a chiral intermediate of omarigliptin. The method comprises the following steps: carrying out amidation, alder condensation, hydrazine hydrate condensation, rearrangement, Boc addition, ring opening and Grubbs catalyst ring formation on starting raw materials including crotonyl chloride and (S)-4-benzyl-2-oxazolidone and carrying out hydrolysis to obtain the chiral intermediate (IX). The preparation method disclosed by the invention has the advantages of relatively low cost, easiness for obtaining raw materials and relatively high yield, so that the preparation method is suitable for industrial production. The formula (IX) is shown in the description.
Owner:ANHUI HAIKANG PHARMA
Preparation method of DP-IV inhibitor omarigliptin intermediate
ActiveCN109651203ASimple post-processingHigh purityCarbamic acid derivatives preparationOrganic compound preparationSulfonateCarbamate
The invention provides a preparation method of a DP-IV inhibitor omarigliptin intermediate. The preparation method includes the step that a raw material A which is 2-(2,5-difluorophenyl)-(2-oxoethyl)carbamate reacts with a raw material B which is alkyl propargyl sulfonate, so that a product C which is 1-(2,5- difluorophenyl)-1-oxopent-4-yne-2-yl carbamate is obtained. The preparation method is new, the specific omarigliptin intermediate is selected as a problem-solving direction, an efficient technology is provided, and the yield of a one-step reaction is up to 96%.
Owner:HENAN MEDICAL COLLEGE
Preparation method for omarigliptin midbody
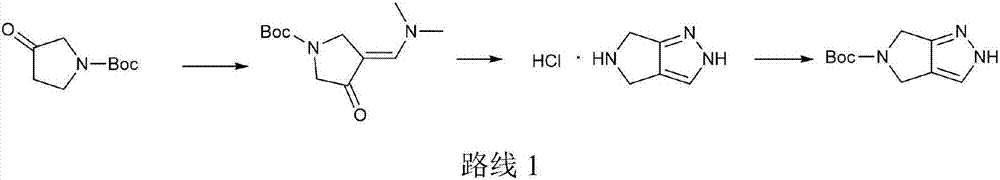
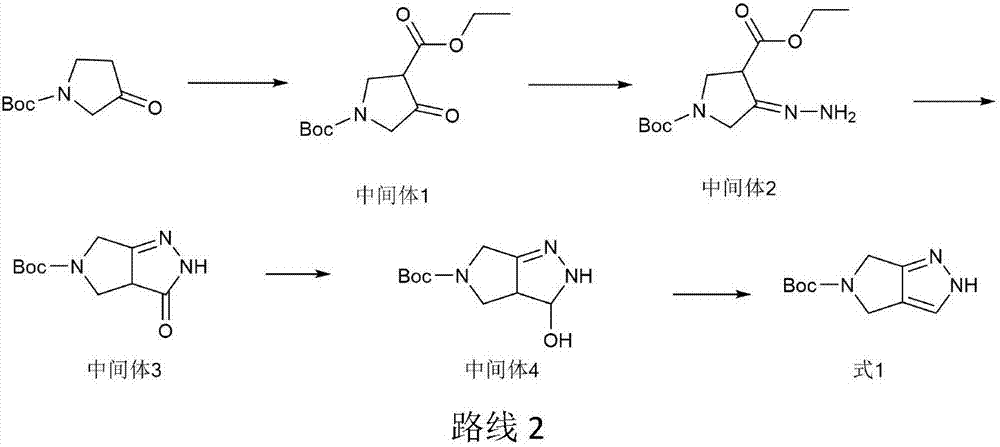
The invention provides an efficient and simple preparation method for omarigliptin midbody pyrrole and [3,4-c] pyrazol-5(2H,4H,6H)-carboxylic acid tert-butyl ester, wherein the total recovery ratio is close to 50%. The preparation method is easy to operate, the recovery ratio is higher, three produced wastes are less, and the method is suitable for industrialized production.
Owner:济南同路医药科技发展有限公司
A kind of dpp-4 inhibitor and its preparation and application in diabetes
The invention discloses a compound or pharmaceutically acceptable salt thereof shown as a formula I (the formula l is shown in the following description), wherein R1, R2 and R3 are each independentlyselected from H or OCH3. In an in-vitro DDP-4 enzyme inhibition test, IC50 values of listed compounds to DDP-4 are all smaller than omarigliptin and Sitagliptin. The test shows that the compound provided by the invention has better DDP-4 inhibition activity and can be used for deeper study as a drug for treating and / or preventing non-insulin-dependent diabetes mellitus, hyperglycemia or insulin resistance.
Owner:中昱医学检验(广州)有限公司
Omarigliptin refining method
InactiveCN107827894ASimple methodEasy to operateOrganic active ingredientsOrganic chemistryHexamethylphosphoramidePhosphoramide
The invention relates to a method for refining alogliptin, which belongs to the technical field of preparation of raw materials. The technical scheme of the present invention is: dissolve the crude product of alogliptin in hexamethylphosphoramide; add a certain amount of activated carbon, stir, and filter; The mixed solution of base phosphoramide-acetone, white solid is separated out, continue stirring for 5-8 hours; Filter, filter cake is beaten with isopropanol of the same volume as the first step, stir for 1 hour; Filter, filter cake is with isopropanol After washing, the filter cake was spread out and dried under vacuum at 25°C. The invention provides a method for refining alogliptin. The method of the invention is simple, easy to operate and suitable for industrial production.
Owner:WEIHAI GUANBIAO INFORMATION TECH
Preparation method of chiral intermediate of omarigliptin
ActiveCN107459501AStable in natureRaw materials are easy to getOrganic chemistry methodsSynthesis methodsChloride
The invention discloses a novel synthesis method for preparing a chiral intermediate of omarigliptin. The method comprises the following steps: carrying out amidation, alder condensation, hydrazine hydrate condensation, rearrangement, Boc addition, ring opening and Grubbs catalyst ring formation on starting raw materials including crotonyl chloride and (S)-4-benzyl-2-oxazolidone and carrying out hydrolysis to obtain the chiral intermediate (IX). The preparation method disclosed by the invention has the advantages of relatively low cost, easiness for obtaining raw materials and relatively high yield, so that the preparation method is suitable for industrial production. The formula (IX) is shown in the description.
Owner:钟桂发
Synthesis process of omarigliptin
ActiveCN111793071AHigh synthetic yieldReduce generationOrganic chemistryBiochemical engineeringChemical compound
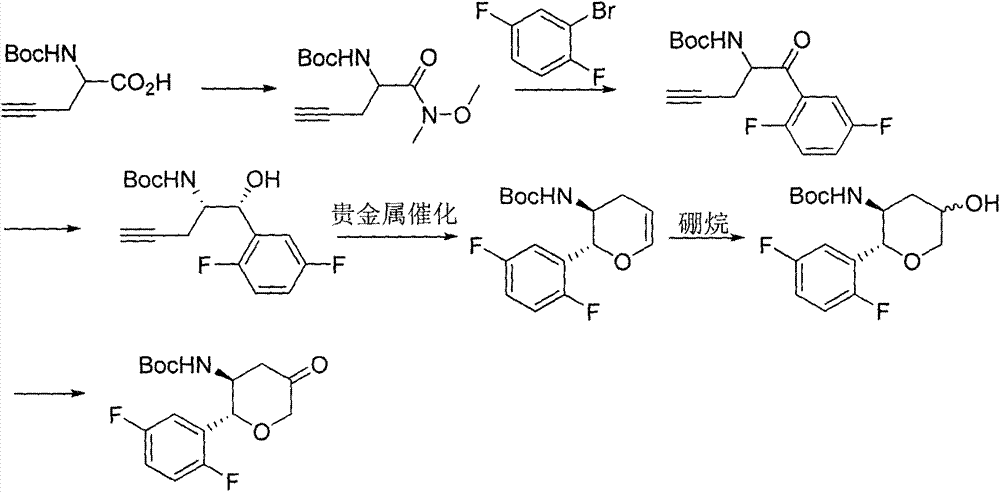
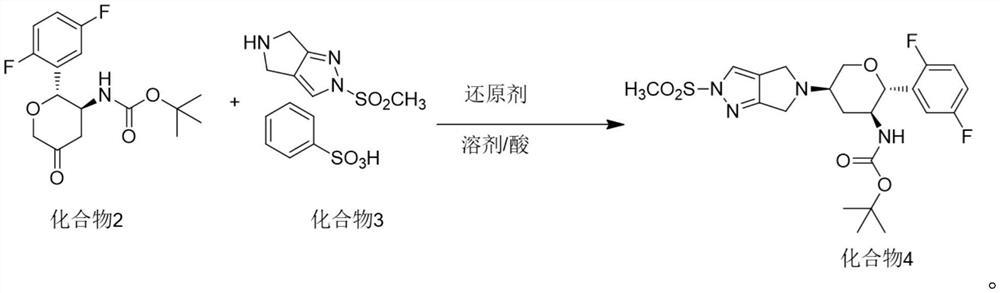
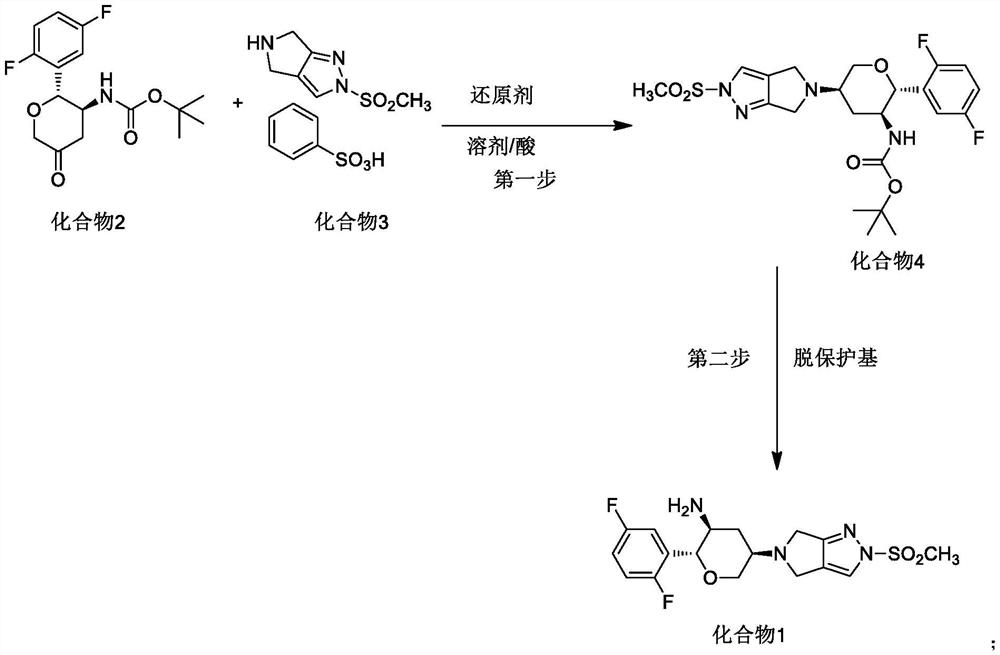
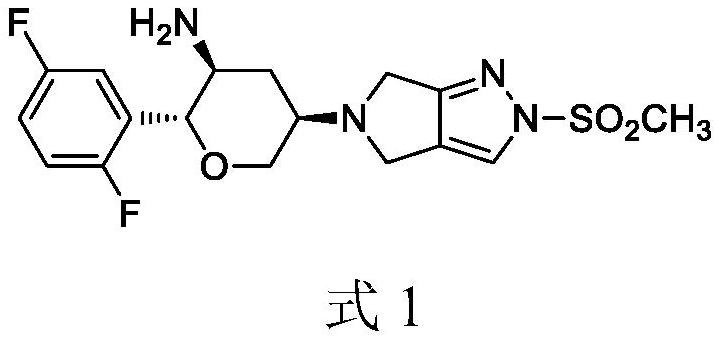
The invention discloses a method for preparing omarigliptin and a method for preparing an intermediate. In the method for preparing the key intermediate compound 4 of the omarigliptin, a weak acid with the pKa value of 3.5-5 is added, so that the generation of byproducts is effectively inhibited, the yield of the intermediate compound 4 is very high, and finally, the yield of the omarigliptin is greatly improved.
Owner:四川凯科医药科技有限公司
The preparation method of dp-iv inhibitor alogliptin intermediate
ActiveCN109651203BSimple post-processingHigh purityCarbamic acid derivatives preparationOrganic compound preparationPolymer scienceAlogliptin
Owner:HENAN MEDICAL COLLEGE
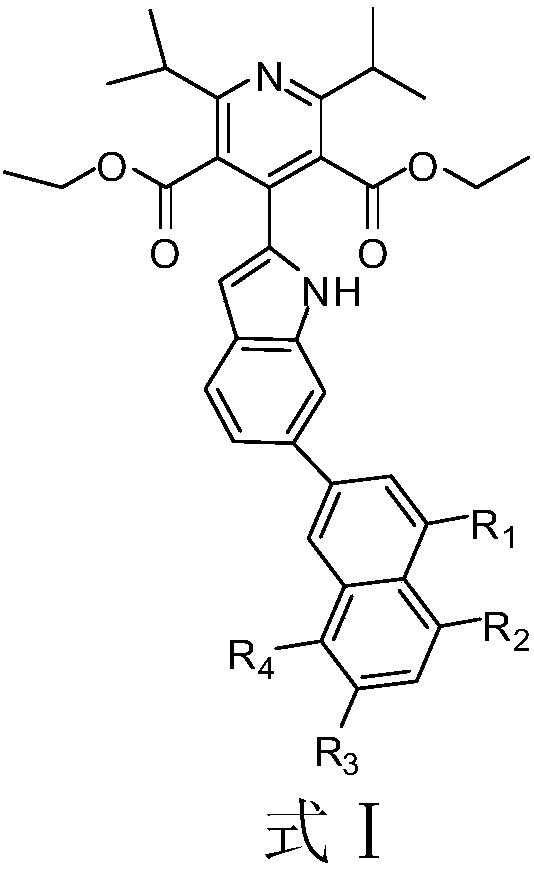
Indole derivative and application of indole derivative in diabetes mellitus
InactiveCN108484573AOrganic active ingredientsOrganic chemistryAcute hyperglycaemiaEnzyme inhibition
The invention discloses an indole derivative represented by a formula I (as shown in the description) or a pharmaceutically acceptable salt of indole derivative, wherein R1, R2, R3 and R4 are respectively selected from H or CH3. The inhibition activity of a compound listed in an in-vitro DPP-4 enzyme inhibition test to DPP-4 is between the inhibition activities of Sitagliptin and omarigliptin. Theindole derivative represented by the formula I is a novel-structure DPP-4 inhibitor, the features of the indole derivative in pharmacology toxicology and pharmacokinetics are worthy of being relatively deeply researched, and a drug for treating and / or preventing non-insulin-dependent diabetes mellitus, hyperglycemia or insulin resistance can be obtained.
Owner:王先化
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com