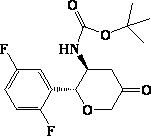
New method for preparing omarigliptin key intermediate
An intermediate and key technology are applied in the field of preparation of key intermediates of aulogliptin, can solve the problems of low synthesis yield, expensive catalyst and high cost, and achieve the advantages of few synthesis steps, avoiding column chromatography separation operation and short time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
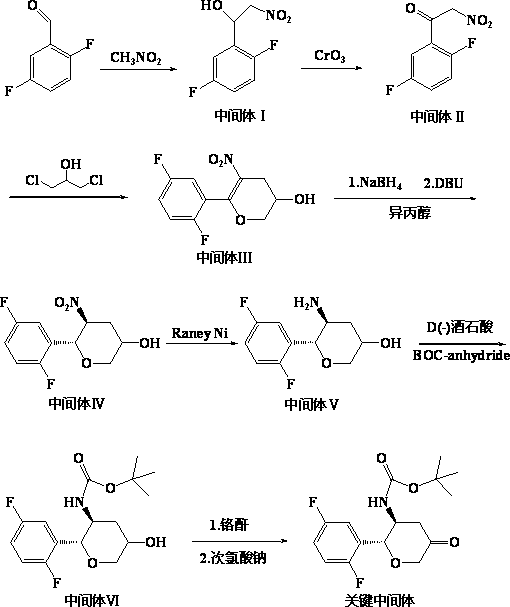
[0022] Embodiment 1: the preparation method of alogliptin intermediate.
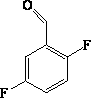
[0023] Dissolve 31.2g (0.78mol) of NaOH in a mixture of water and methanol, cool the ethanol refrigerant to 0°C, and add 57.3ml (0.52mol) of 2,5-difluorobenzaldehyde and 42.6ml of nitromethane ( 0.78mol) of methanol solution, neutralized with glacial acetic acid after the reaction, added ethyl acetate and stirred, then layered, the organic phase was washed successively with sodium carbonate solution and saturated brine, then dried with anhydrous sodium sulfate, filtered, concentrated, 112g of 2-nitro-1(2,5-difluorophenyl)-ethanol (intermediate I) was obtained, with a yield of 97% and an HPLC of 91.4%. 1 H NMR (500MHz, CDCl 3 ), δ (ppm): 6.88 (m, 3H), 4.76 (d, 2H), 4.5 (m, 1H), 2.0 (d, 1H).
[0024] Dissolve 100g (0.45mol) of intermediate I in 200ml of acetone and cool down to 0°C, add dropwise 90g (0.9mol) of Jones reagent, keep the temperature of the reaction solution at 3°C, after the completion of t...
Embodiment 2
[0030] Embodiment 2: the preparation method of alogliptin intermediate.
[0031] Dissolve 15.6g (0.39mol) NaOH in a mixture of water and methanol, cool the ethanol refrigerant to 0°C, and add 28.8ml (0.26mol) of 2,5-difluorobenzaldehyde and 21.3ml of nitromethane ( 0.39mol) of methanol solution, neutralized with glacial acetic acid after the reaction, added ethyl acetate and stirred, then layered, the organic phase was washed successively with sodium carbonate solution and saturated brine, then dried with anhydrous sodium sulfate, filtered, concentrated, 55.9 g of 2-nitro-1(2,5-difluorophenyl)-ethanol (intermediate I) was obtained, with a yield of 98% and an HPLC of 92.6%. 1 H NMR (500MHz, CDCl 3 ), δ (ppm): 6.88 (m, 3H), 4.76 (d, 2H), 4.5 (m, 1H), 2.0 (d, 1H).
[0032] Dissolve 50g (0.23mol) of intermediate I in 100ml of acetone and cool down to 0°C, add 46g (0.46mol) of Jones reagent dropwise, the temperature of the reaction solution is maintained at 2°C, after the complet...
Embodiment 3
[0038] Embodiment 3: the preparation method of alogliptin intermediate.
[0039] Dissolve 46.8g (1.17mol) of NaOH in a mixture of water and methanol, cool the ethanol refrigerant to 0°C, and add 86.4ml (0.78mol) of 2,5-difluorobenzaldehyde and 63.9ml of nitromethane ( 1.17mol) of methanol solution, neutralized with glacial acetic acid after the reaction, added ethyl acetate and stirred, then layered, the organic phase was washed successively with sodium carbonate solution and saturated brine, then dried with anhydrous sodium sulfate, filtered, concentrated, 164 g of 2-nitro-1(2,5-difluorophenyl)-ethanol (intermediate I) was obtained, with a yield of 96% and an HPLC of 92.7%. 1 H NMR (500MHz, CDCl 3 ), δ (ppm): 6.88 (m, 3H), 4.76 (d, 2H), 4.5 (m, 1H), 2.0 (d, 1H).
[0040] Dissolve 149g (0.68mol) of intermediate I in 300ml of acetone and cool down to 0°C, add dropwise 137g (1.36mol) of Jones reagent, the temperature of the reaction solution is maintained at 3°C, after the com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com