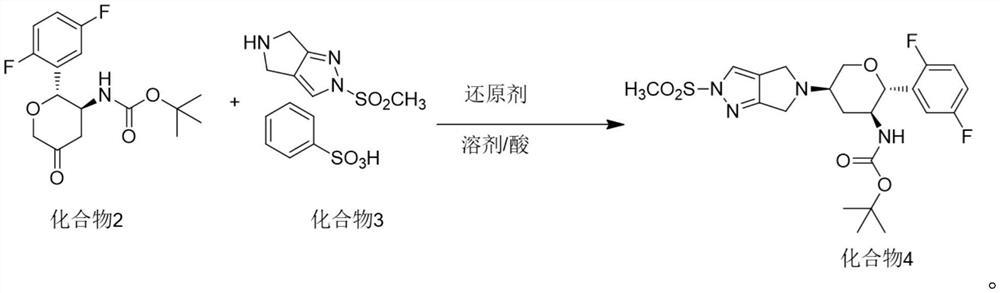
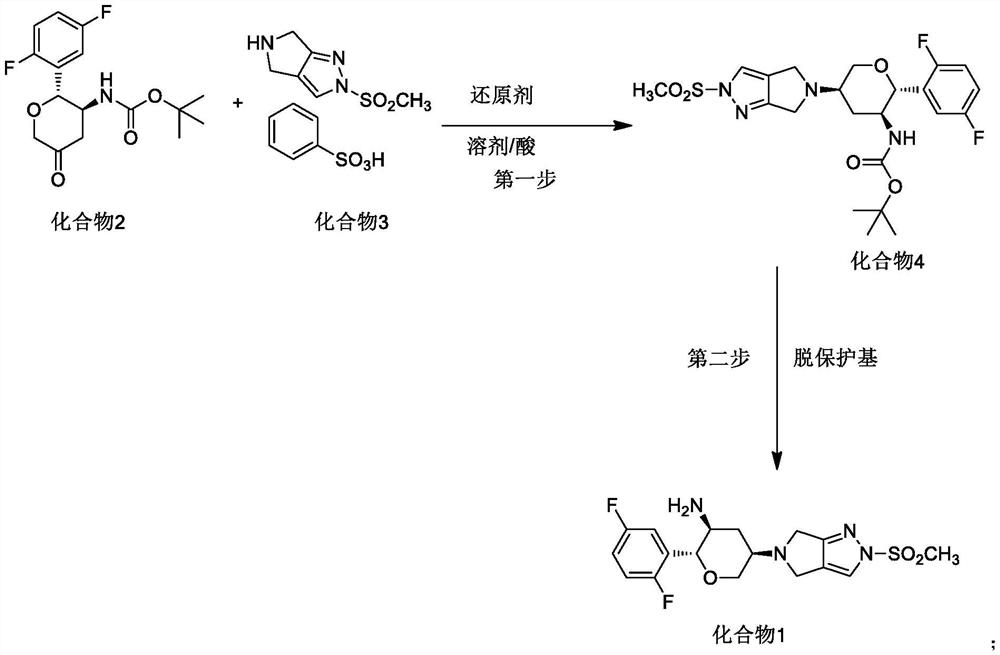
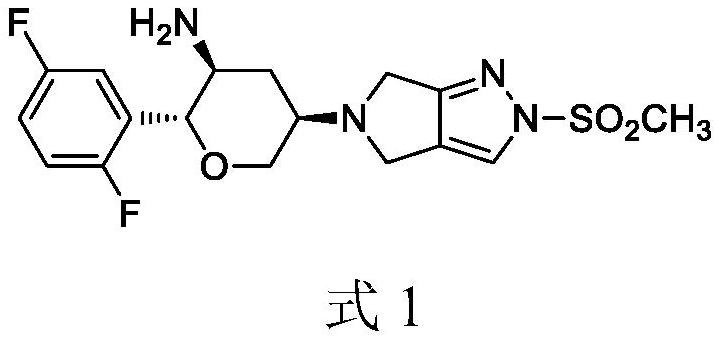
Synthesis process of omarigliptin
A compound and solvent technology, which is applied in the field of preparation of the compound alogliptin, can solve the problems of low reaction yield and high synthesis cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] Take 8.25g of compound 2, 9.8g of compound 3, 120ml of DMA and 40ml of acetic acid, cool to -15°C in an ice-salt bath, then slowly add 6.9g of sodium triacetylborohydride, after the reaction, add 200ml of water After stirring, the precipitated solid was filtered and dried to obtain 11.74 g of compound 4.
[0048] Add 190 ml of dichloromethane and 9.05 g of benzenesulfonic acid to the reaction vessel, add 11.7 g of compound 4 in batches with stirring, after the reaction is completed, add 190 ml of water, discard the organic phase, and add 100 ml of dichloromethane to the water phase Then slowly add 10.3 grams of sodium bicarbonate, separate the dichloromethane phase, extract the aqueous phase with dichloromethane again, combine the dichloromethane, add ethyl acetate to crystallize after concentration, filter and dry to obtain the final augliptin product 7.52 grams, purity greater than 99.0%, high resolution MS: m / z=399.1298 (M+1).
Embodiment 2
[0050] Take 8.25g of compound 2, 9.8g of compound 3, 120ml of DMA and 40ml of propionic acid, cool to -15℃ in an ice-salt bath, then slowly add 6.9g of sodium triacetylborohydride, after the reaction, add 200ml of water After stirring, the precipitated solid was filtered and dried to obtain 11.42 g of compound 4.
[0051] Add 180 ml of dichloromethane and 8.54 g of benzenesulfonic acid to the reaction vessel, add 11.42 g of compound 4 in batches under stirring. After the reaction is completed, add 180 ml of water, discard the organic phase, and add 100 ml of dichloromethane to the water phase. Then slowly add 9.7 grams of sodium bicarbonate, separate the dichloromethane phase, extract the aqueous phase again with dichloromethane, combine the dichloromethane, add ethyl acetate to crystallize after concentration, filter and dry to obtain the final augliptin product 7.17 g, purity greater than 99.0%, high resolution MS: m / z=399.1298 (M+1).
Embodiment 3
[0053] Take 8.25g of compound 2, 9.8g of compound 3, 120ml of DMA and 40ml of acetic acid, cool to -15℃ in an ice-salt bath, then slowly add 1.3g of sodium borohydride, after the reaction is completed, add 200ml of water, stir and filter The precipitated solid was dried to obtain 11.43 g of compound 4.
[0054] Add 190 ml of dichloromethane and 8.8 g of benzenesulfonic acid to the reaction vessel, add 11.43 g of compound 4 in batches with stirring, after the reaction is completed, add 190 ml of water, discard the organic phase, and add 100 ml of dichloromethane to the water phase Then slowly add 10.0 g of sodium bicarbonate, separate the dichloromethane phase, extract the aqueous phase with dichloromethane again, combine the dichloromethane, add ethyl acetate to crystallize after concentration, filter and dry to obtain the final augliptin product 7.24 grams, purity greater than 99.0%, high resolution MS: m / z=399.1298 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com