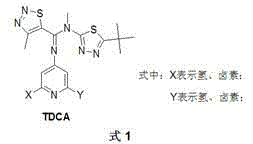
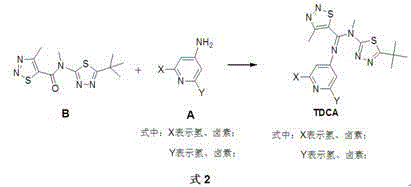
Multi-nitrogen heterocycle thiadiazoles-5-formamidine compound by cyclization method
A technology of compound and target compound, which is applied in the field of synthesis of 1,2,3-thiadiazole-5-carboxamidine compounds, can solve the problems of large spatial position, low reactivity, and complex components, so as to reduce the amount of production, The effect of good product quality and high synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
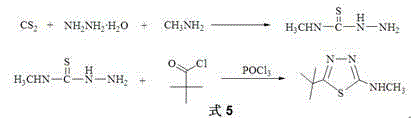
[0042] Synthesis of 4-methyl-1,2,3-thiadiazole-5-carbonyl chloride (compound Q ):
[0043] 1) Synthesis of ethyl carbazate:
[0044] Add 540ml (4.4mol) of diethyl carbonate and 255g (4mol) of 80% hydrazine hydrate into a 2000ml three-necked round bottom flask equipped with a condensing reflux tube, stir, and gradually raise the temperature to 50°C, the reaction system changes from turbid to clear liquid, keep the temperature and stir for 1 hour, lower to room temperature and continue to stir for 24 hours, distill ethanol and water under reduced pressure (remove the water brought in by ethanol and hydrazine hydrate), and dry in vacuum to obtain 399.2 g of white solid product, which is directly processed Next reaction.
[0045] 2) Synthesis of ethyl 3-(ethoxycarbonyl-hydrazone)butanoate
[0046] Add 508 mL (4 mol) of ethyl acetoacetate into a 2000ml three-neck flask, stir magnetically, and add 399.2 g of ethyl carbazate dropwise over 2 hours under ice water cooling (prepared ...
Embodiment 2
[0053] Synthesis of N-methyl-4-methyl-1,2,3-thiadiazole-5-carboxamide (compound P ):
[0054] Add 162.6 g (1.00 mol) of 4-methyl-1,2,3-thiadiazole-5-carbonyl chloride (compound Q ), 37.3 g (1.20 mol) methylamine, 1000 mL toluene. Heat to 100°C, absorb the hydrogen chloride gas produced by the reaction with lye, react for 10 h, cool the reaction system to room temperature, pour the reaction solution into an equal volume of water, and adjust the pH value of the obtained mixture with 2% sodium hydroxide aqueous solution, Make it pH=7. Separate the liquid to obtain a toluene solution containing N-methyl-4-methyl-1,2,3-thiadiazole-5-carboxamide, distill and concentrate the toluene solution to obtain a brown solid N-methyl-4-methyl- The crude product of 1,2,3-thiadiazole-5-carboxamide was 155.6 g, the liquid phase normalized purity was 97.9%, and the yield was 99%. The crude brown solid N-methyl-4-methyl-1,2,3-thiadiazole-5-carboxamide does not need further purification and can ...
Embodiment 3
[0056] Synthesis of N'-(2,6-difluoropyridin-4-yl)-N,4-dimethyl-1,2,3-thiadiazole-5-carboxamidine (compound N-10 ) (X=F, Y=F in Formula 8):
[0057]
[0058] Under the condition of nitrogen protection, 200ml of anhydrous sulfolane, 2,6-difluoro-4-aminopyridine (13.0g, 100mmol), compound P (N-methyl-4-methyl-1 , 2,3-thiadiazole-5-carboxamide) (15.7g, 100mmol), add freshly distilled thionyl chloride (35.7g, 300mmol), DCC (dicyclohexylcarbodiimide) (20.7g, 100mmol ) after heating to 75°C for 12 hours, the reaction was changed from a reflux device to a distillation device, and the low-boiling components in the reactor were distilled (the main component was unreacted thionyl chloride), and the temperature was raised to 100°C for 3 hours, and then stopped reaction. Cool slightly, pour the reaction solution into a large amount of crushed ice, adjust pH=7 with 1M aqueous sodium hydroxide solution, filter with suction, and recrystallize the obtained product with a mixed solvent of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com