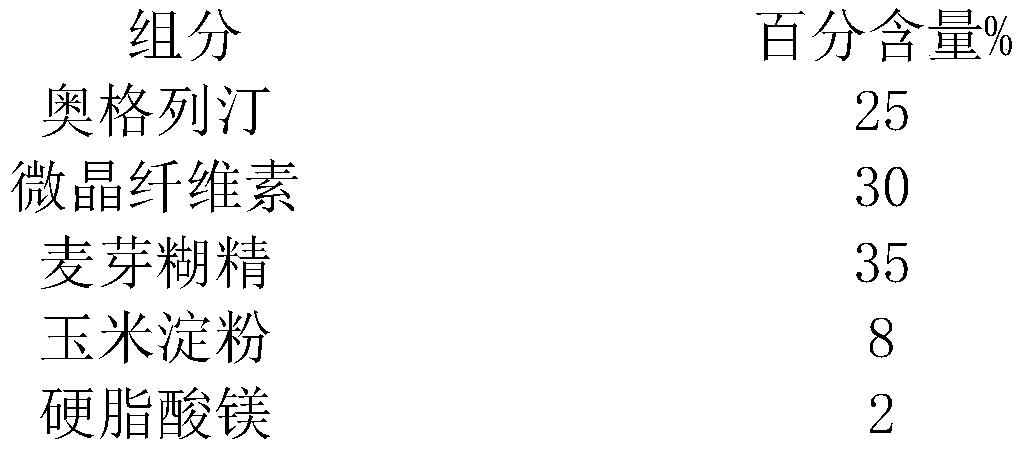DP-IV inhibitor drug composition
A technology of 1. DP-IV, composition, applied in the direction of drug combination, pharmaceutical formula, medical preparation of non-active ingredients, etc., can solve the problems of difficulty in localization, large fluctuation of sustained release, non-equivalence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: Compatibility of raw and auxiliary materials
[0018] Mix alogliptin with cornstarch and maltodextrin respectively, and examine their compatibility. Under the accelerated condition of 50°C / 75%RH, after 2 weeks, it is found that the content of total degradation impurities does not exceed 0.5%. Meet the pharmaceutical requirements.
Embodiment 2
[0019] Embodiment 2: prescription screening
[0020] Compress the material amount of 100 tablets as shown in the table below (each tablet contains 25mg of API):
[0021] Table 1 Prescription Screening
[0022]
[0023]
[0024] Preparation Process:
[0025] (1) Pass the above-mentioned excipients and API through 80 and 100 mesh sieves respectively, and set aside.
[0026] (2) Mix the API and all the excipients except magnesium stearate uniformly by equal addition method.
[0027] (3) continue to add magnesium stearate, fully mix.
[0028] (4) Press into plain tablets, investigate the disintegration time limit of the obtained samples, the results are shown in Table 2.
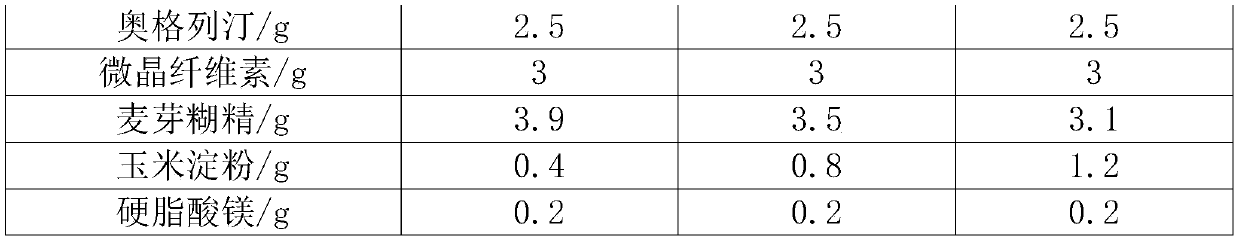
[0029] Table 2
[0030]
[0031] Obviously, the disintegration of formulation 1 is too slow, which is not conducive to dissolution.
Embodiment 3
[0032] Example 3: Prescription scaling up
[0033] Carry out scale-up research according to preparation 2, 3 prescription, each batch of each prescription is pressed 1000 tablets, and process is with reference to embodiment 2, the result finds:
[0034] Formulation 3 has poor fluidity, and the problem of compressibility is more obvious, and its friability is as high as 5%, while the friability of formulation 2 is only 8‰.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com