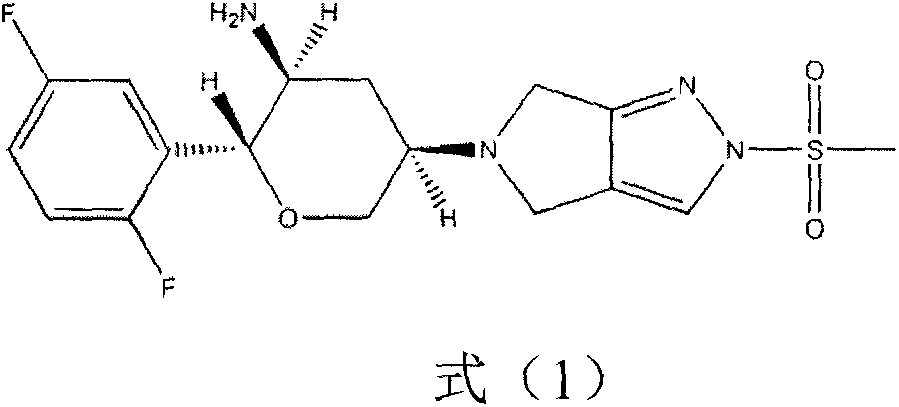
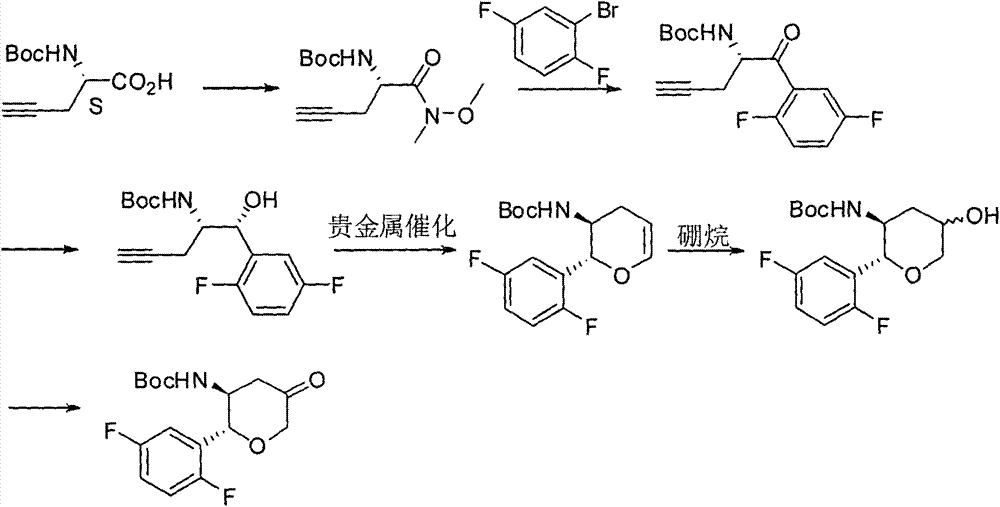
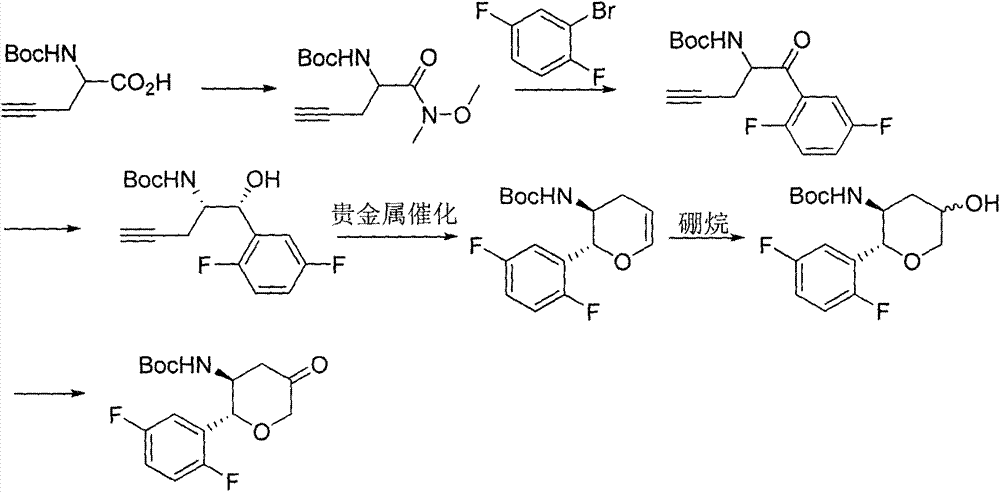
Preparation method of chiral intermediate of omarigliptin
A technology of chiral intermediates and intermediates, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of high cost, unavailable raw materials, and low yield, and achieve the effects of stable properties, novel routes, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The synthesis of embodiment 1 intermediate (I)
[0041]
[0042] Dissolve 20g (0.123mol) (S)-4-benzyl-2-oxazolidinone in 200ml of dichloromethane, add 25.6ml (0.184mol) of triethylamine and 0.45g (0.0037mol) of 4-dimethylamino pyridine. With stirring in an ice bath at 0°C, (15.4 g, 0.15 mol) crotonyl chloride was added dropwise. Maintained at 0°C and stirred for 3h, TLC showed that the raw material had disappeared, added dichloromethane and water, separated the organic layer, dried, concentrated, and obtained intermediate (I) by silica gel column chromatography, 26.8g, yield: 89%, [M +H + ]: 246.1.
Embodiment 2
[0043] The synthesis of embodiment 2 intermediate (II)
[0044]
[0045] Method 1: Replace the nitrogen reaction bottle, add 24.5g (0.1mol) of intermediate (I) and 163ml of anhydrous dichloromethane, lower the temperature to -5°C, and slowly add 32.9g (0.12mol) of trifluoroform Dibutylboron sulfonate, stirred for 10 minutes after dropping. Then 21 ml (0.15 mol) of triethylamine were added dropwise. After the dropwise addition was completed, the temperature was lowered to -70° C., and a solution of 15 g (0.105 mol) of 2,5-difluorobenzaldehyde in 50 ml of dichloromethane was slowly added dropwise. The temperature rose to -10°C over 1 hour and stirred for 1 hour. TLC shows that the reaction is complete, adding 102ml of potassium phosphate buffer successively, 53ml of methyl alcohol and 53ml of 35% hydrogen peroxide, separating the organic layer, washing with saturated sodium bicarbonate, washing with sodium thiosulfate, drying, and separating the intermediate (II) by silica ...
Embodiment 3
[0047] The synthesis of embodiment 3 intermediate (III)
[0048]
[0049] 19.3g (0.05mol) of intermediate (II) was dissolved in 150ml of tetrahydrofuran, and 3.6ml (0.0735mol) of hydrazine hydrate was slowly added dropwise under stirring in an ice bath at 0°C. Maintain stirring at 0 ° C for 2h. After TLC detection showed that the reaction was complete, ethyl acetate and water were added. The organic layer was separated, and the aqueous layer was extracted twice with ethyl acetate. The organic layers were combined, dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and separated by silica gel column chromatography to obtain 11.4 g of intermediate (III), with a yield of 94%. , [M+H + ]: 243.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com