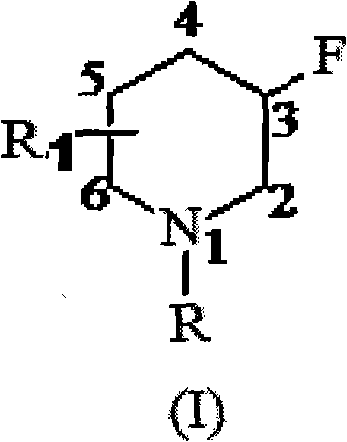
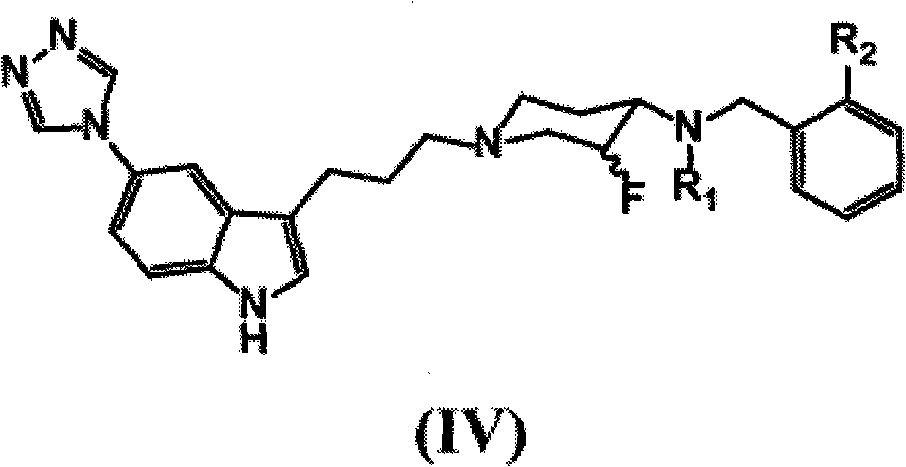
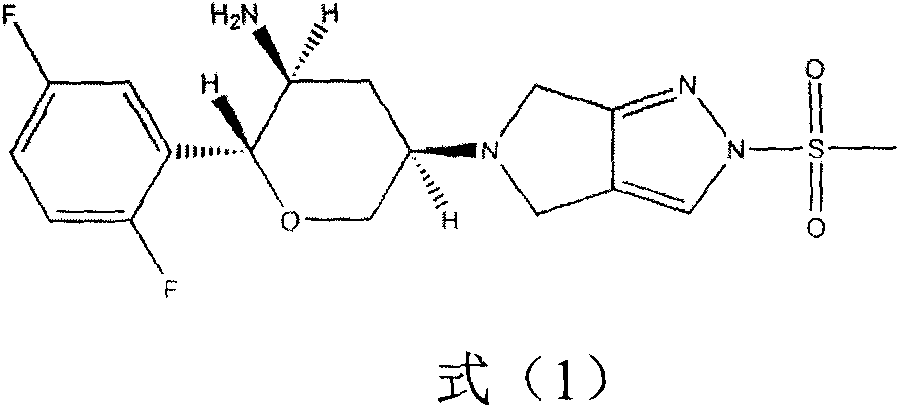
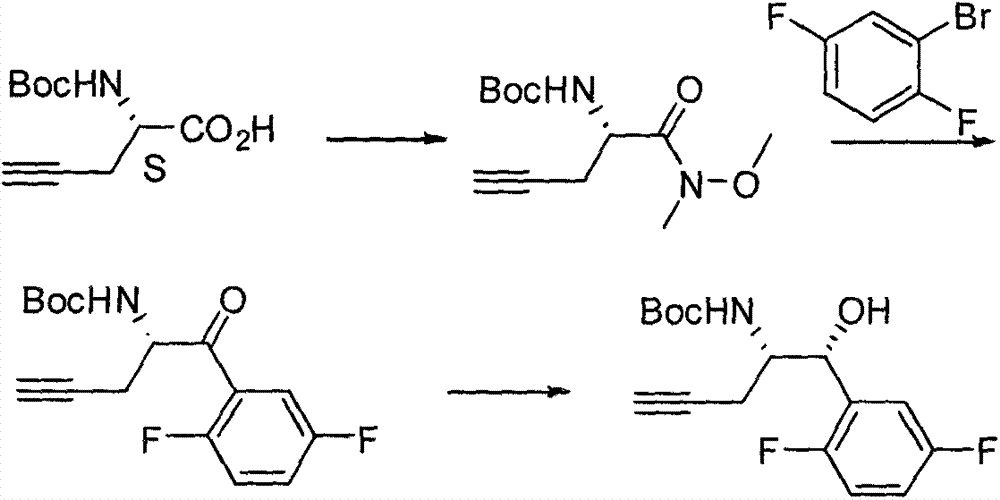
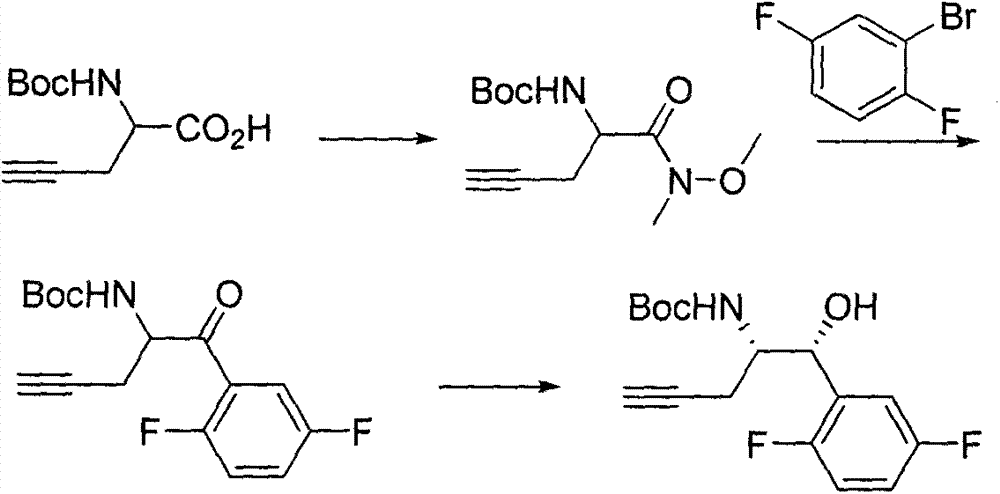
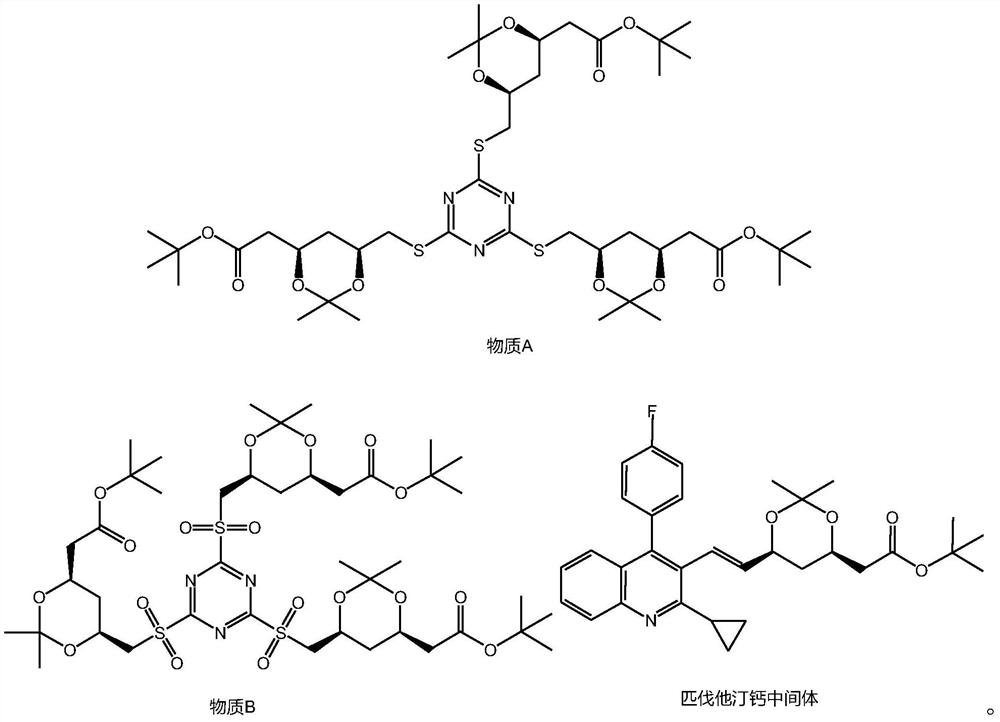
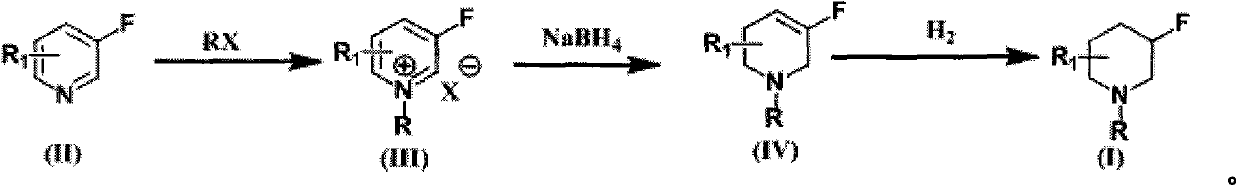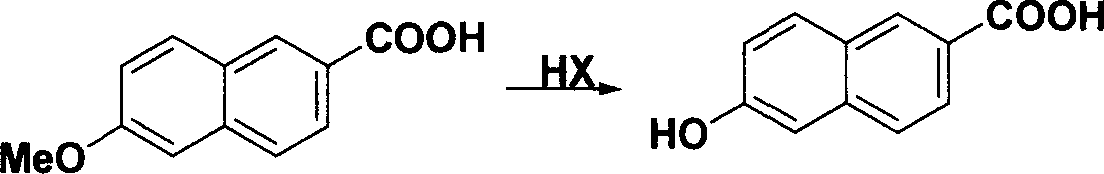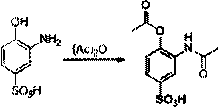Patents
Literature
56results about How to "New route" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
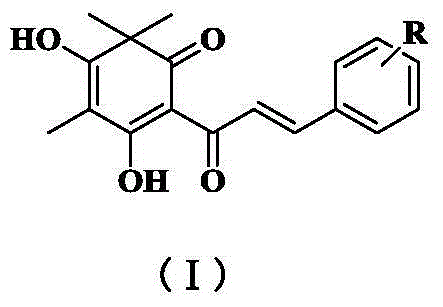
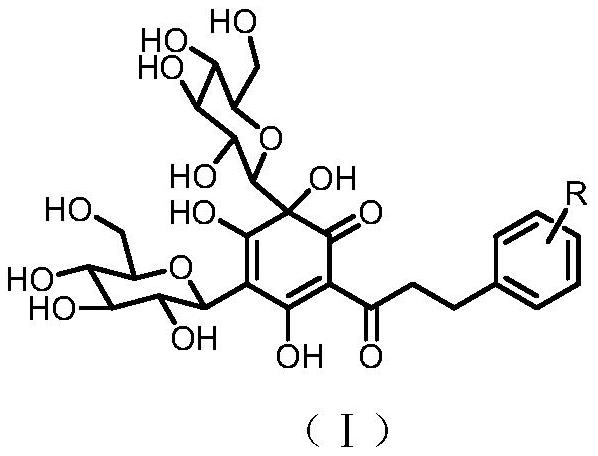
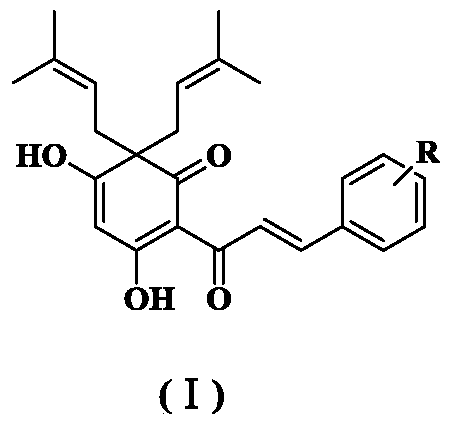
Quinoid chalcone compound with methyl group at A ring, and preparation method and anti-inflammatory activity thereof
ActiveCN105085218ANovel structureNew routeOrganic active ingredientsNervous disorderMethyl groupAnti-inflammatory analgesics
The invention discloses a quinoid chalcone compound with a methyl group at an A ring, and a preparation method and anti-inflammatory activity thereof. The compound has a structure as shown in a general formula (I) which is described in the specification. The preparation method comprises the following steps: (1) synthesizing 2-hydroxy-4,6-dimethoxyacetophenone; (2) synthesizing 2'-hydroxy-4',6'-dimethoxychalcone derivative; (3) synthesizing 2',4',6'-trihydroxy chalcone derivative; and (4) synthesizing the quinoid chalcone compound with a methyl group at the A ring. The compound is simple to prepare and has obvious anti-inflammatory action.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
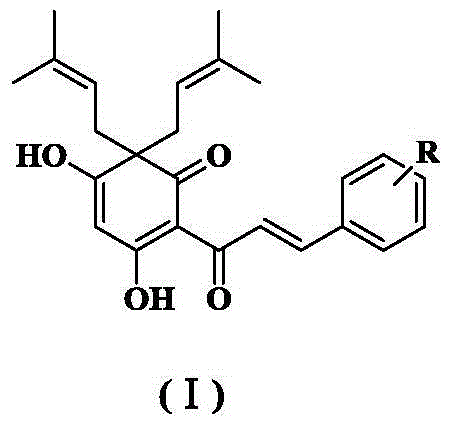
Quinoid chalcone compound with isopentenyl group at A ring, and preparation method and anti-inflammatory activity thereof
ActiveCN105085219ANovel structureNew routeOrganic active ingredientsNervous disorderChalconeAnti-inflammatory analgesics
The invention discloses a quinoid chalcone compound with an isopentenyl group at an A ring, and a preparation method and anti-inflammatory activity thereof. The compound has a structure as shown in a general formula (I) which is described in the specification. The preparation method comprises the following steps: (1) synthesizing 3,5-dihydroxy-2-acetyl-6,6-diisopentenylcyclohexyl-2,4-dienone; (2) synthesizing 3-hydroxy-5-methoxy-2-acetyl-6,6-diisopentenylcyclohexyl-2,4-dienone; (3) synthesizing a methoxy group protected quinoid chalcone compound with the isopentenyl group at the A ring; and (4) synthesizing the quinoid chalcone compound with the isopentenyl group at the A ring. The compound is simple to prepare and has obvious anti-inflammatory action.
Owner:稷冲(北京)医药有限公司
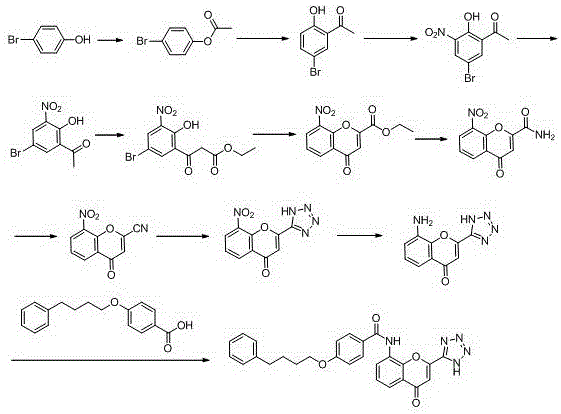
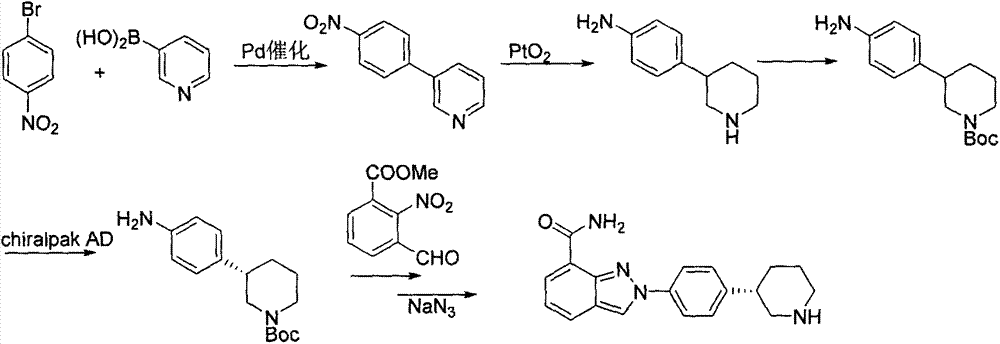
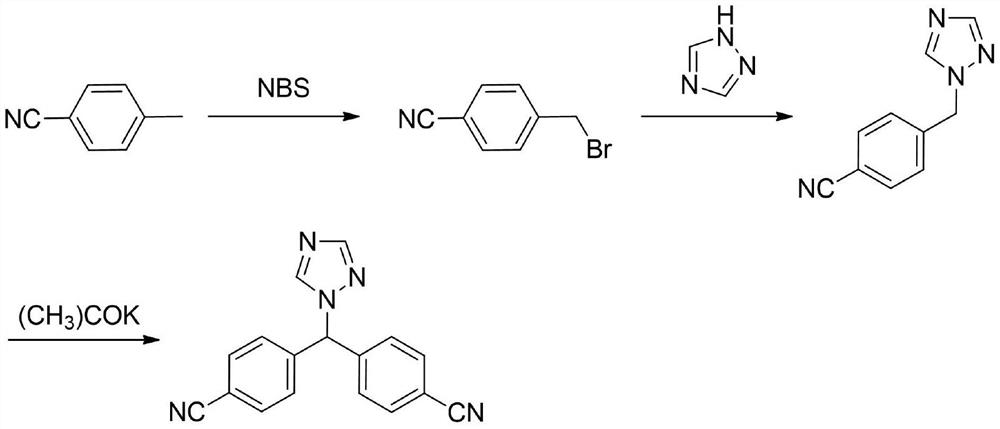
New preparation method of Pranlukast
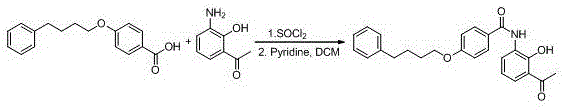
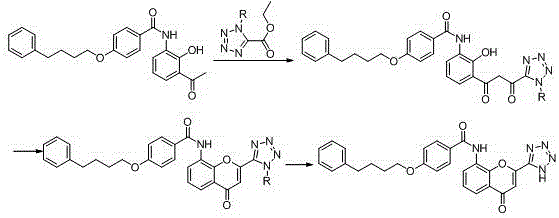
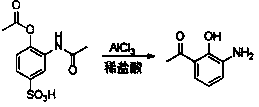
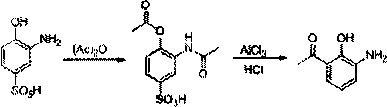
The invention provides a new preparation method of drug Pranlukast for treating asthma. The new preparation method includes the specific steps that with 2-aminophenol-4-sulfonic acid as a starting material, a key intermediate 3-amino-2-hydroxyacetophenone is prepared by means of acylation, Fries rearrangement and deprotection, then reacts with 4-(phenylbutoxy)benzoic acid, and then is subjected to condensation with ethyl 1H-tetrazole-5-acetate, and finally preparation is achieved through ring closing under the acidic condition. Compared with the prior art, the raw material used for the new preparation method is low in price and easy to obtain, industrialization of a process can be achieved easily, and the obtained final product is high in purity; and no dangerous process exists, equipment is simple, and the route is novel.
Owner:上海微巨实业有限公司
Method for preparing tapentadolhydrochloride and intermediate thereof
InactiveCN102002065AThe market is well suppliedLow priceGroup 4/14 element organic compoundsAmino preparation from aminesEnantiomerCombinatorial chemistry
The invention relates to a method for preparing a tapentadolhydrochloride and an intermediate thereof, in particular to a compound shown in a structural formula II, an enantiomer, a diastereoisomer and a raceme of the compound, a mixture of the enantiomer and the diastereoisomer and salts thereof, a method for preparing a compound shown in the formula II, application of the compound shown in the formula II in preparation of the tapentadolhydrochloride, and an intermediate for preparing the compound shown in the formula II. The method for preparing the tapentadolhydrochloride has the advantages of mild condition, simplicity of operation, high stereoselectivity, security, environment friendliness, suitability for large-scale commercial production, and abundant and low-cost raw material and catalyst.
Owner:TOPHARMAN SHANGHAI
3-droperidol derivative and preparation method thereof
InactiveCN102344407ASolve choice problemsResolve separation difficultiesOrganic chemistryArylHalogen
The invention provides a 3-droperidol derivative represented by a structural formula (I), wherein R is hydrogen, C1-C6 alkyl, aryl, benzyl, or a structure as the formula; R1 is hydrogen, C1-C18 alkyl, aryl, benzyl or hydroxymethyl; R2 is C1-C6 alkyl, aryl, benzyl or alkoxy; substitution positions of R2 on a benzene ring are 2-, 3-, 4-, 5-, 6- sites or a plurality of sites; and the previous groups are not substituted or substituted by one or more substituents selected from alkyl, alkyl halide, hydroxyalkyl, halogen, alkoxy, or hydroxyl. The invention also provides a preparation method of the 3-droperidol derivative. The preparation method is novel, the raw materials and the reagents are cheap, the reaction conditions are mild, and the operations are easy. With the method, produced 1-substituent-3-droperidol can be used as an important intermediate in novel medicine developing.
Owner:SHANGHAI AQ BIOPHARMA CO LTD
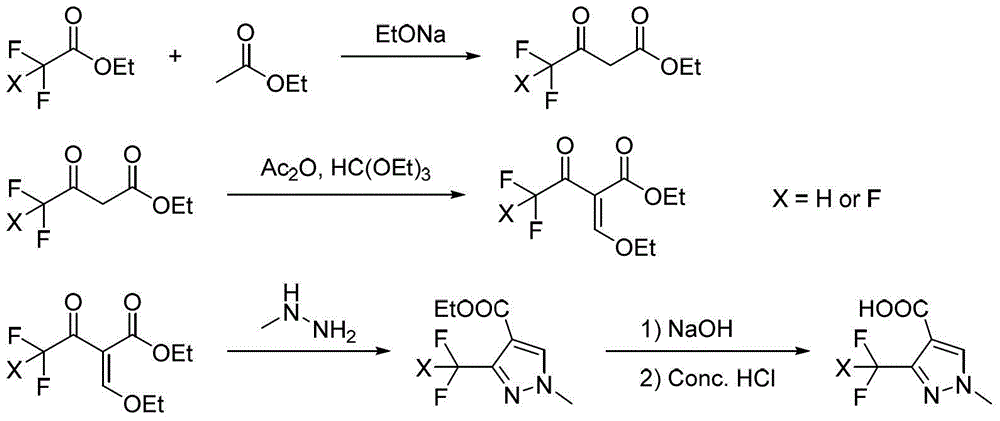
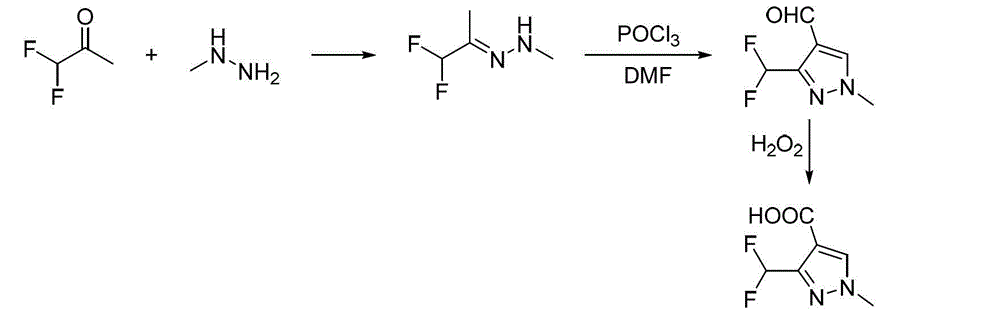
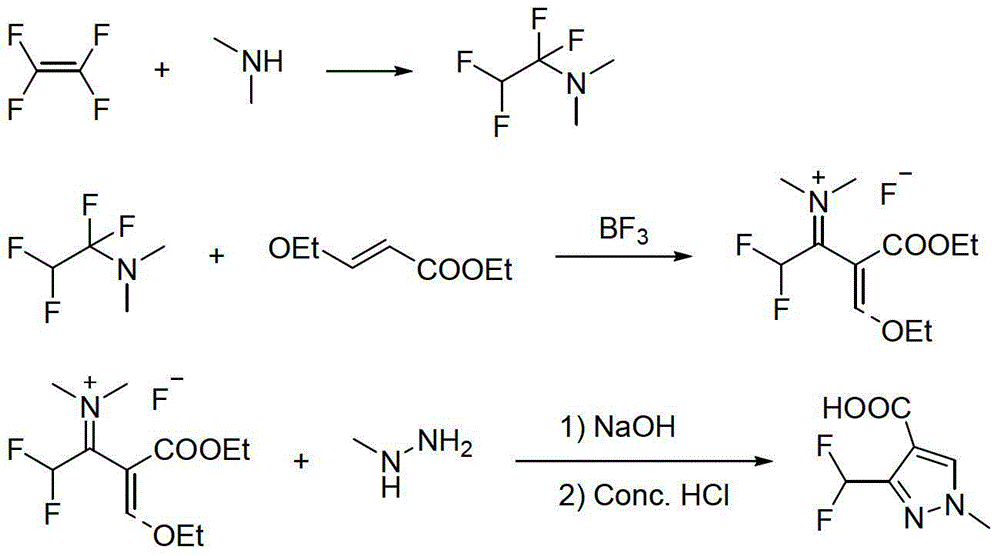
Preparation method for polyfluoromethylpyrazole compound, and intermediate of compound and preparation method thereof
The invention discloses a preparation method for a polyfluoromethylpyrazole compound, and an intermediate of the compound and a preparation method thereof. The invention provides a preparation method for a compound 1 as defined in the specification. The preparation method comprises a step of subjecting a compound 2 as defined in the specification and methylhydrazine to a ring-closure reaction in an organic solvent so as to obtain the compound 1, wherein R<1> is a C1-4 alkyl group, R<2> is a methyl or ethyl group and x is 2 or 3. The preparation method provided by the invention uses cheap and easily available raw materials and is mild in reaction conditions, safe to operate, environment friendly, low in production cost, high in reaction conversion rate, low in the content of isomer in by-products, high in reaction yield and product purity and suitable for industrial production.
Owner:LIANHE CHEM TECH YANCHENG +4
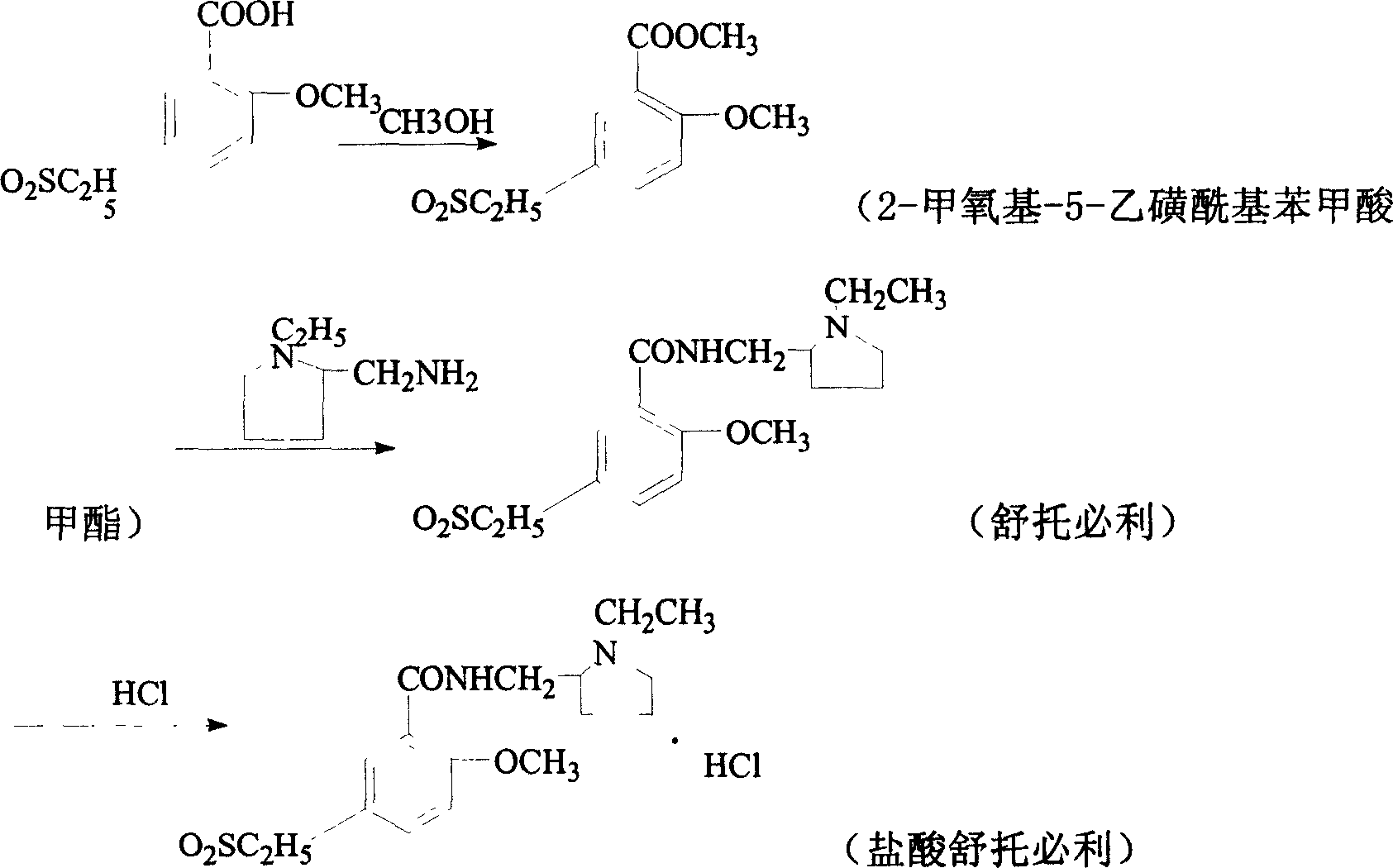
New synthesis process of sultopride hydrochloride
The present invention relates to the new synthesis process of sultopride hydrochloride. The synthesis process includes the following steps: 1. the reaction between 2-methoxy-5-ethanesulbonyl benzoic acid and methanol to produce 2-methoxy-5-ethanesulbonyl methyl benzoate; 2. the reaction between 2-methoxy-5-ethanesulbonyl methyl benzoate obtained in the step 1 and N-ethyl-2-aminomethyl tetrahydropirrolidine to produce sultopride; and 3. the reaction between sultopride and hydrochloric acid to obtain sultopride hydrochloride.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
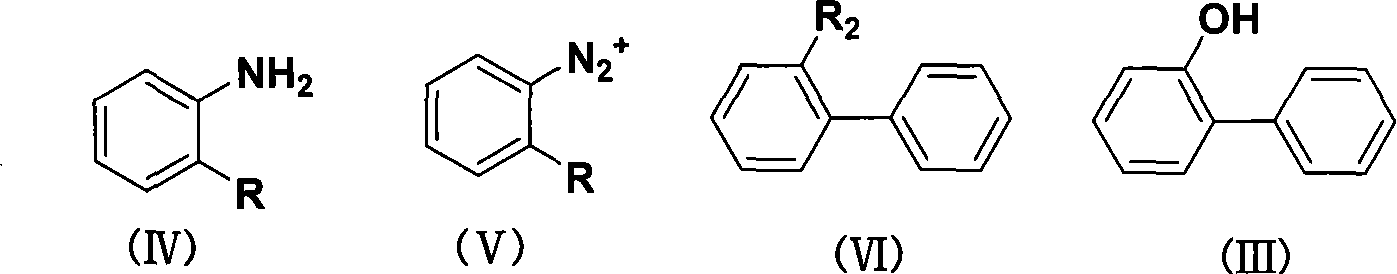
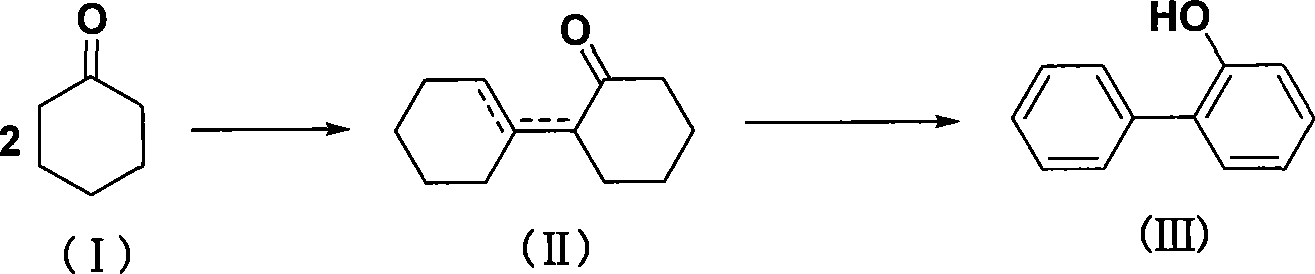
Method for synthesizing o-phenylphenol
InactiveCN101062887ANew routeLow costOrganic chemistryOrganic compound preparationBenzeneO-Phenylphenol
The invention discloses a synthesizing method of orthoxenol with amino benzenes compound, which comprises the following steps: choosing amino benzenes compound as raw material; diazotizing; coupling; generating diphenyl compound; hydrolyzing; getting the orthoxenol. This invention possesses simple operation, low cost and high receiving ratio, which adopts a new route to proceed.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Rhodococcus ZJPH1003 and application thereof in preparing S-(+)-2,2-dimethylcyclopropane carboxylic acid
ActiveCN102161978AEasy to trainReduce manufacturing costBacteriaMicroorganism based processesMicroorganismCarboxylic acid
The invention provides a new strain-Rhodococcus sp. ZJPH1003 and an application thereof in preparing S-(+)-2,2-dimethylcyclopropane carboxylic acid through microbial catalytic asymmetric hydrolysis of 2,2-dimethylcyclopropane ethyl formate. The strain is conserved at China Center for Type Culture Collection, the conservation address is Wuhan University, Wuhan, China, postcode 430072, the conservation number is CCTCC M 2010371, and the conservation date is December 29, 2010. In the invention, the adopted method for preparing the S-(+)-2,2-dimethylcyclopropane carboxylic acid through microbial catalytic asymmetric hydrolysis of the 2,2-dimethylcyclopropane ethyl formate by utilizing the new microbial strain has the advantages of novel route, high yield, environmental friendliness and the like; and by utilizing a chiral biocatalysis method which takes a Rhodococcus ZJPH1003 cell as a catalyst, for the target product-S-(+)-2,2-dimethylcyclopropane carboxylic acid, the e.e can reach 82.5% and the yield can reach 41.3% when the substrate concentration is 30mmol / L.
Owner:ZHEJIANG UNIV OF TECH
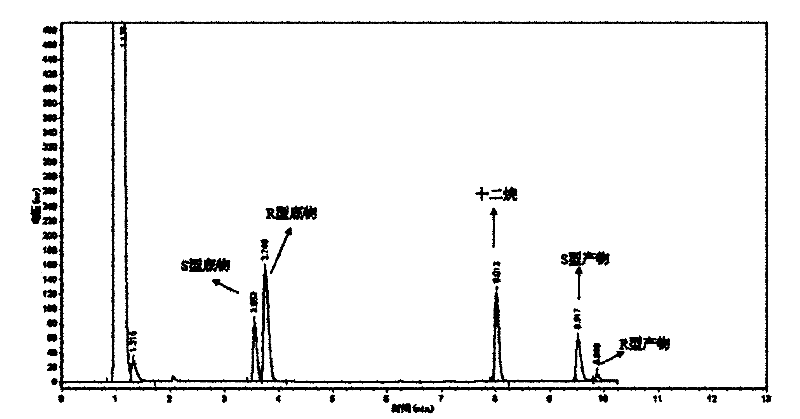
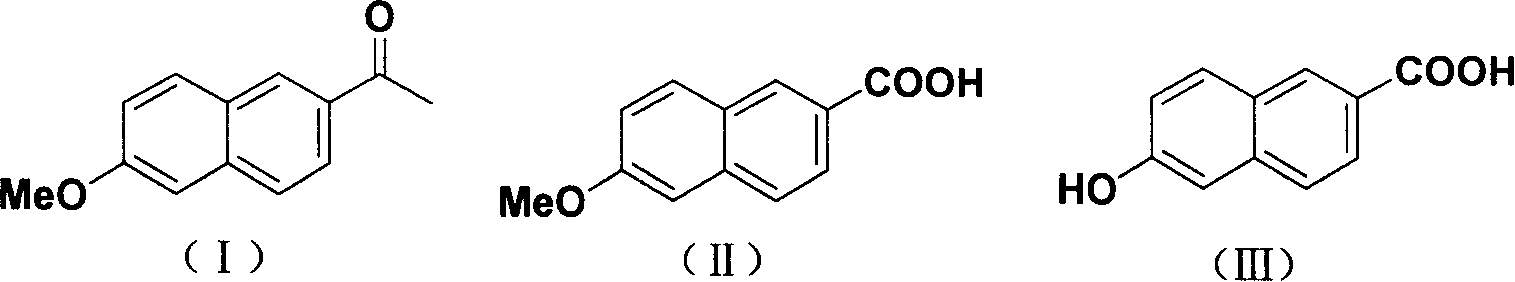
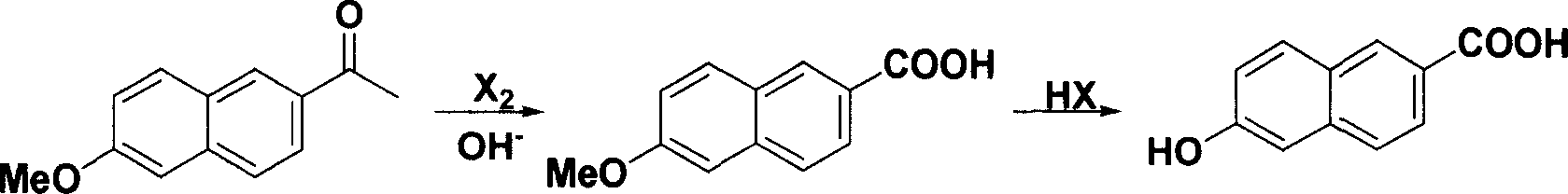
Method for synthesizing 6-hydroxy-2-naphthoic acid
InactiveCN1844072ANew routeEasy to operateOrganic compound preparationCarboxylic compound preparationHalogenAcetic acid solution
The invention provides a process for preparing 6- hydroxyl-2- naphthoic acid, which comprises the steps of: reacting compound of formula (I) with halogens under alkaline condition at 0-100 deg C for 7-12hr to obtain compound of formula (II), then reacting compound of formula (II) with HX in acetic acid solution at 70 deg C to backflow temperature to obtain compound of formula (III). The invention can realize high purity and high yield.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Preparation method of larotinib intermediate, and intermediate compound
PendingCN111793016AStable in natureRaw materials are cheap and easy to getCarbamic acid derivatives preparationOrganic compound preparationBiochemical engineeringTert-Butyloxycarbonyl protecting group
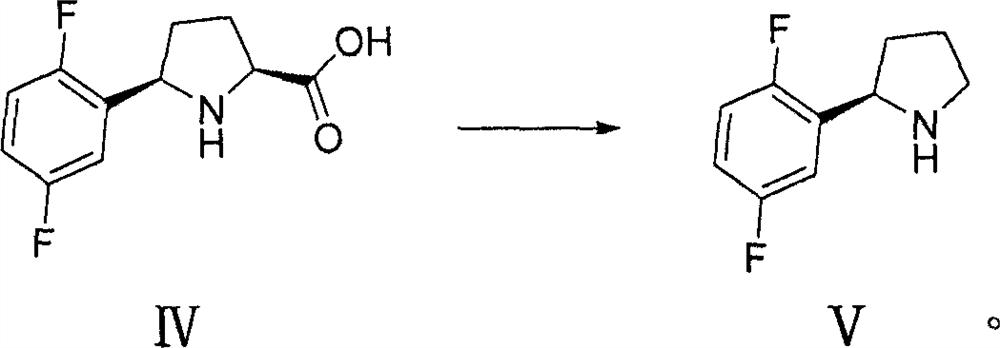
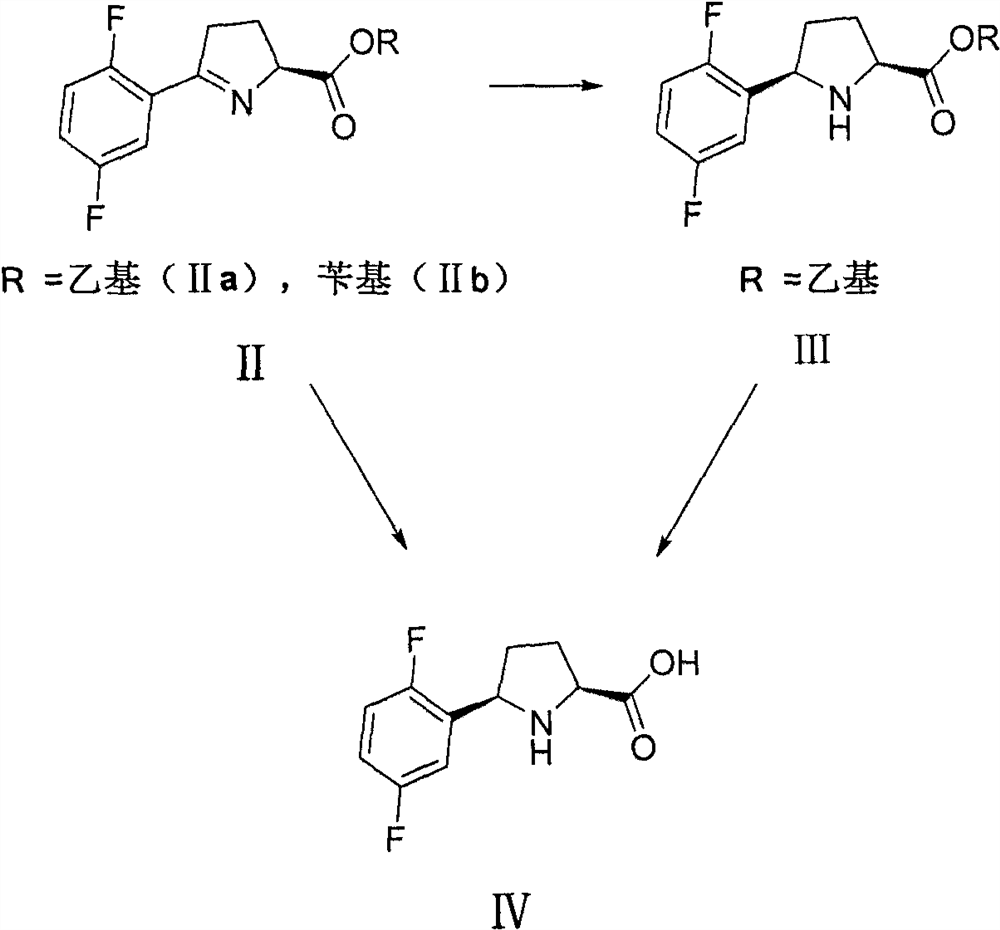
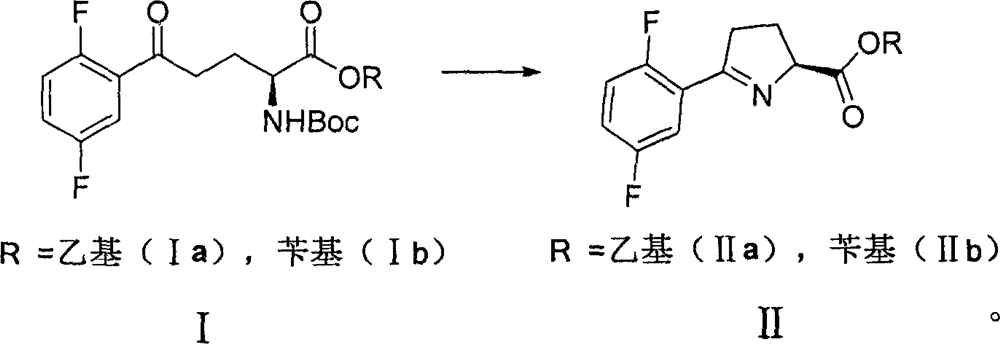
The invention discloses a preparation method of a larotinib intermediate represented by V, and an intermediate compound. The method comprises the following steps: taking 2,5-difluorobromobenzene and N-tert-butoxycarbonyl-L-pyroglutamate as initial raw materials, and carrying out coupling, deprotection group cyclization, reduction and decarboxylation to obtain a compound represented by a formula V.According to the invention, the preparation method has the advantages of novelty, low cost, cheap and easily available raw materials and high yield, and is suitable for large-scale industrial production.
Owner:钟桂发
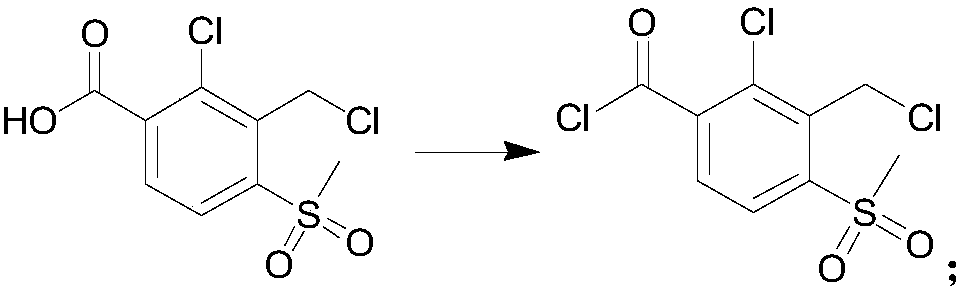
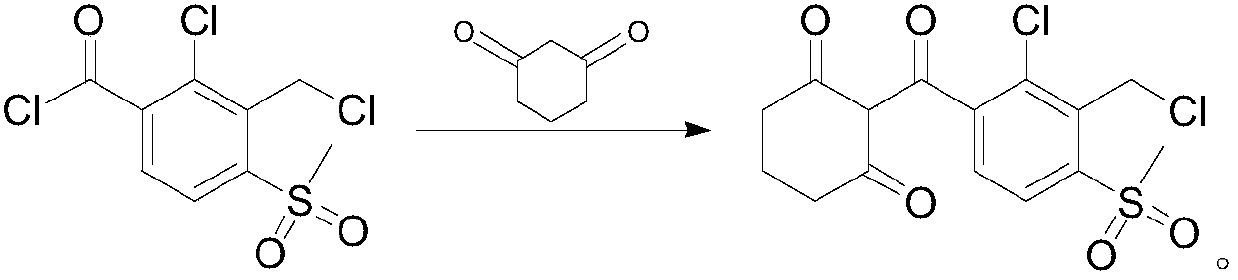
Preparation method of 2-(2-chloro-3-chloromethyl-4-methylsulfonyl benzoyl)-1,3-cyclohexanedione
InactiveCN110357797ANo generationImprove developmentOrganic chemistryOrganic compound preparationBenzoic acidSynthesis methods
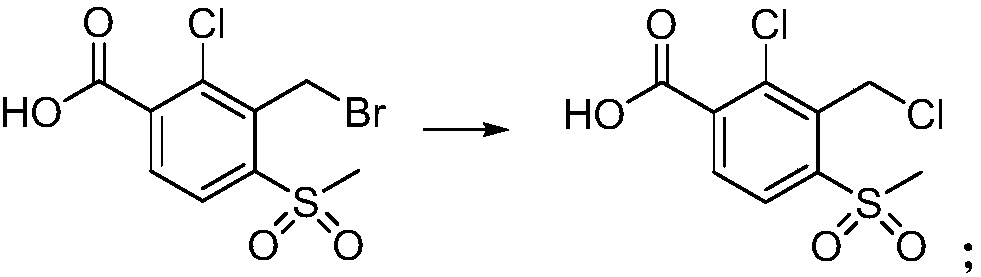
The invention provides a preparation method of 2-(2-chloro-3-chloromethyl-4-methylsulfonyl benzoyl)-1,3-cyclohexanedione. The preparation method comprises the following steps that 2-chloro-3-bromomethyl-4-methylsulfonyl benzoic acid is taken as a raw material, through chlorination, acyl chlorination and 1,3-cyclohexanedione substitution and rearrangement reaction, and the 2-(2-chloro-3-chloromethyl-4-methylsulfonyl benzoyl)-1,3-cyclohexanedione is obtained. According to the preparation method, the new synthesis method is provided, the final yield can reach more than 90%, the HPLC detection content can reach more than 99%, purity is high, and the preparation method can be used as a reference substance, is conducive to development of the analysis method of tembotrions, more beneficial to quality control of tembotrions products, and provides a new thinking for the quality control of the products.
Owner:JIANGXI TIANYU CHEM CO LTD
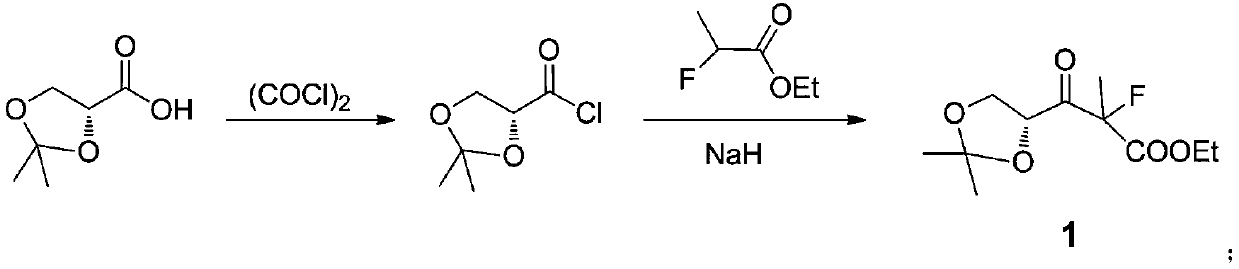
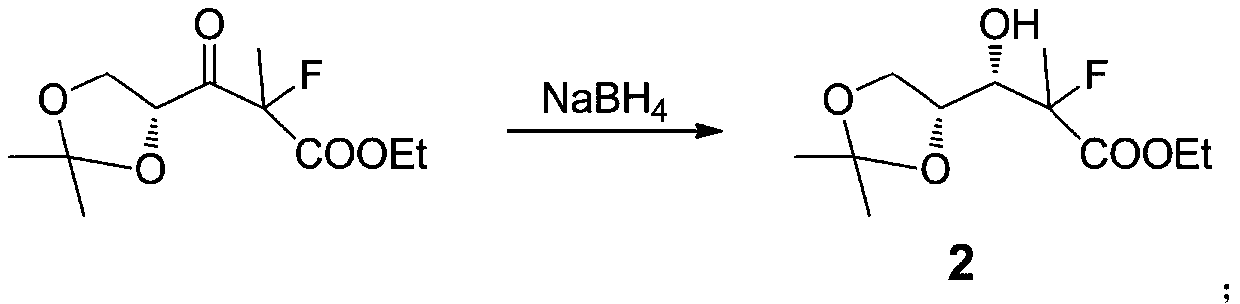
Preparation method of sofosbuvir key intermediate
ActiveCN111362989ACheap and easy to getHigh purityEsterified saccharide compoundsSugar derivativesBenzoic acidPropanoic acid
The invention discloses a preparation method of a sofosbuvir key intermediate ((2R,3R,4R,5R)-3-(benzoyloxy)-5-chloro-4-fluoro-4-methyltetrahydrofuran-2-yl)benzoic acid methyl ester. The preparation method comprises the following steps: by using (R)-2,2-dimethyl-1,3-dioxolane-4-carboxylic acid as a starting material, performing acylating chlorination, performing a reaction with alpha-fluoropropionic acid, performing carbonyl reduction, performing hydroxyl protection, performing hydrolytic cyclization, performing hydroxymethyl protection, performing reduction, and performing chlorination to prepare the sofosbuvir key intermediate ((2R,3R,4R,5R)-3-(benzoyloxy)-5-chloro-4-fluoro-4-methyltetrahydrofuran-2-yl)benzoic acid methyl ester. The preparation scheme has a short synthetic route and a high yield, and avoids a fluorination reaction step in the synthetic process.
Owner:IANGSU COLLEGE OF ENG & TECH
Rhodococcus ZJPH1003 and application thereof in preparing S-(+)-2,2-dimethylcyclopropane carboxylic acid
ActiveCN102161978BReduce manufacturing costHigh catalytic efficiencyBacteriaMicroorganism based processesPtru catalystCarboxylic acid
The invention provides a new strain-Rhodococcus sp. ZJPH1003 and an application thereof in preparing S-(+)-2,2-dimethylcyclopropane carboxylic acid through microbial catalytic asymmetric hydrolysis of 2,2-dimethylcyclopropane ethyl formate. The strain is conserved at China Center for Type Culture Collection, the conservation address is Wuhan University, Wuhan, China, postcode 430072, the conservation number is CCTCC M 2010371, and the conservation date is December 29, 2010. In the invention, the adopted method for preparing the S-(+)-2,2-dimethylcyclopropane carboxylic acid through microbial catalytic asymmetric hydrolysis of the 2,2-dimethylcyclopropane ethyl formate by utilizing the new microbial strain has the advantages of novel route, high yield, environmental friendliness and the like; and by utilizing a chiral biocatalysis method which takes a Rhodococcus ZJPH1003 cell as a catalyst, for the target product-S-(+)-2,2-dimethylcyclopropane carboxylic acid, the e.e can reach 82.5% and the yield can reach 41.3% when the substrate concentration is 30mmol / L.
Owner:ZHEJIANG UNIV OF TECH
Quinoid dihydrochalcone dicarbonyl glycoside compound with glucose on A ring, preparation method and neuroprotective activity thereof
ActiveCN111662261ANovel structureNew routeOrganic active ingredientsOrganic chemistryCerebral ischaemiaMedicinal chemistry
The invention discloses a class of quinoid dihydrochalcone C-glycoside compounds with glucose on a ring A, a preparation method and anti-cerebral ischemia injury activity thereof, wherein the compoundhas a structure represented by a general formula (I). The preparation method of the compound comprises the following steps: (1) synthesizing a 2,4,6-trihydroxy dihydrochalcone compound; (2) synthesizing a 2,4,6-trihydroxy-3,5-diglucosyl dihydrochalcone C-glycoside compound; and (6) synthesizing a class of quinoid dihydrochalcone C-glycoside compound with glucose in the A ring. The compound disclosed by the invention is simple in preparation method and has a remarkable effect of resisting cerebral ischemia injury.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
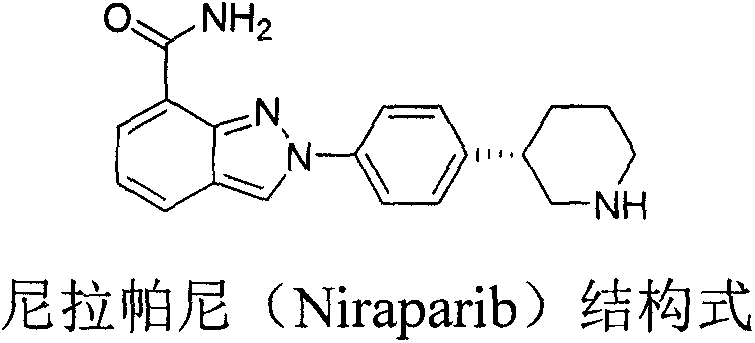
Preparation method of chiral intermediate of niraparib
ActiveCN107311911AStable in natureRaw materials are easy to getOrganic chemistryHydrolysisIntramolecular cyclization
The invention discloses a novel synthetic method for preparing a chiral intermediate of niraparib. The method comprises the following steps: taking 4-bromophenylacetic acid and chiral substituted oxazolone as starting materials; and carrying out amide condensation, Michael addition, hydrolysis, reduction and intramolecular cyclization to obtain the intermediate (VII). The preparation method is low in cost, raw materials are easily obtained, the yield is high, and the synthetic method is suitable for industrialized production.
Owner:钟桂发
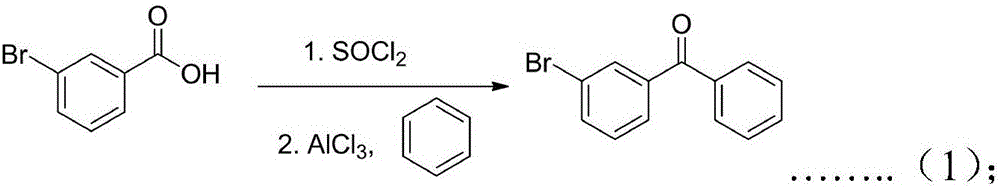
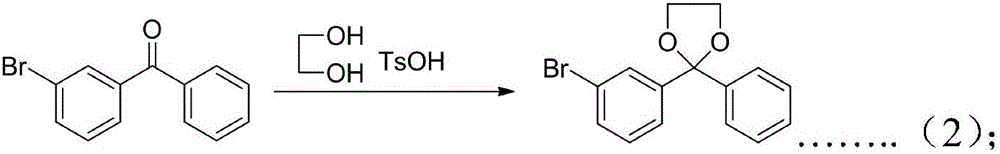
Preparation method of ketoprofen
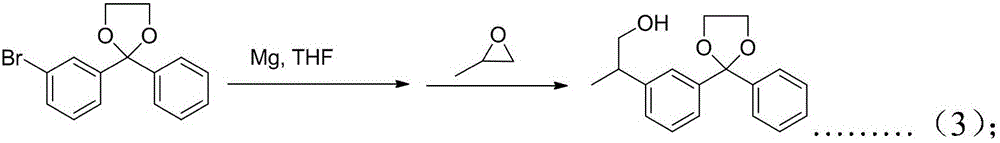
InactiveCN106748723ACheap and easy to getSimple processOrganic compound preparationCarbonyl compound preparation by condensationBenzeneGrignard reagent
The invention discloses a preparation method of ketoprofen. The method takes m-bromobenzoic acid and benzene as starting raw materials, and the ketoprofen is prepared through the steps of acylating, protecting a carbonyl group, taking a Grignard reagent, reacting with propylene oxide, oxidizing, de-protecting and the like. The method disclosed by the invention has the characteristics of short reaction route, easiness of obtaining the raw materials, high yield and low cost.
Owner:IANGSU COLLEGE OF ENG & TECH
Preparation method of m-cyanomethyl methyl benzoate
ActiveCN106928092ACheap and easy to getHigh purityOrganic compound preparationCarboxylic acid esters preparationPhenylacetic acidPhenethyl alcohol
The invention discloses a preparation method of m-cyanomethyl methyl benzoate. According to the method, m-bromobenzoic acid is used as a starting raw material; the m-bromine methyl methyl benzoate is prepared through esterification; m-methoxy formyl phenethyl alcohol is obtained and prepared through Grignard reaction; through oxidization, the m-methoxy formyl phenylacetic acid is prepared; through amidation and dewatering, the m-cyanomethyl methyl benzoate is finally prepared. The preparation method provided by the invention has the advantages that the design is ingenious; the route is novel; the raw materials have low price and can be easily obtained; the process is simple; the implementation is easy; the yield is high; the purity of the obtained final product is high; the quality is high; no dangerous process exists; the extremely toxic substances of cyanides and expensive cyaniding reagents used in the conventional synthesis are avoided; the requirements on the equipment are simple; the production difficulty and the production cost investment are reduced; the industrial production can be favorably realized; the economic benefits are good.
Owner:上海微巨实业有限公司
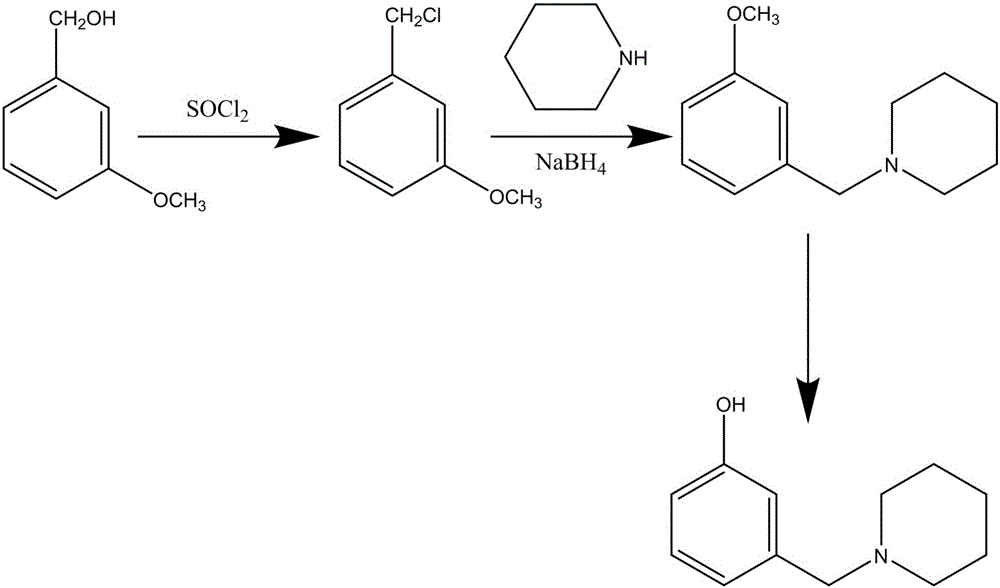
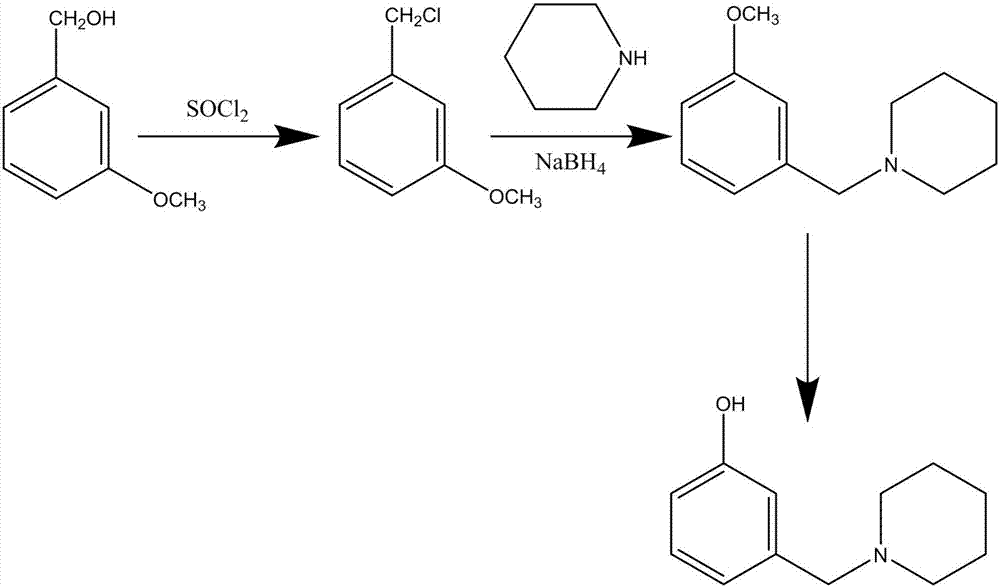
Method for synthesizing Roxatidine intermediate 3-(1-piperidinyl methyl) phenol
The invention discloses a method for synthesizing Roxatidine intermediate 3-(1-piperidinyl methyl) phenol and relates to the technical field of organic synthesis. M-anisyl alcohol which is cheap and easy to obtain is used as a raw material and firstly reacts with sulfinyl chloride to produce m-methoxybenzyl chloride, then the m-methoxybenzyl chloride reacts with piperidine to produce 3-(1-piperidinyl methyl) anisole, and finally demethoxylation is performed in hydrobromic acid to obtain the target product 3-(1-piperidinyl methyl) phenol. The route is clear, the yield is high, the amount of produced waste water, gas and residues is small, the recycling of solvent can be realized, the preparation cost is reduced and the cost is less than half of the cost of the original route.
Owner:ANHUI HERYI CHEM
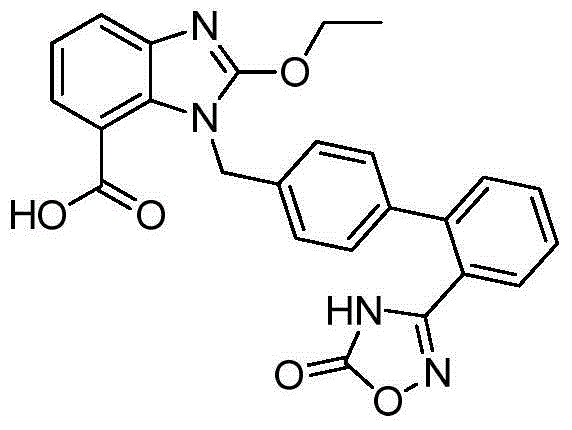
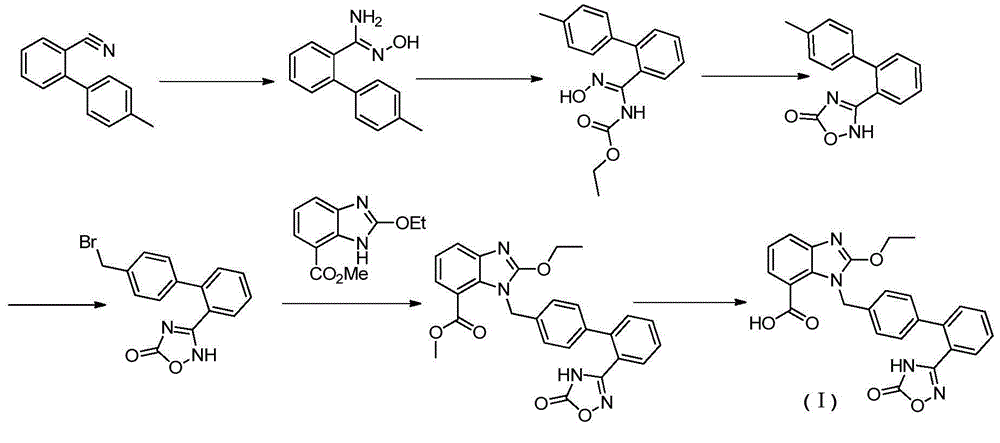
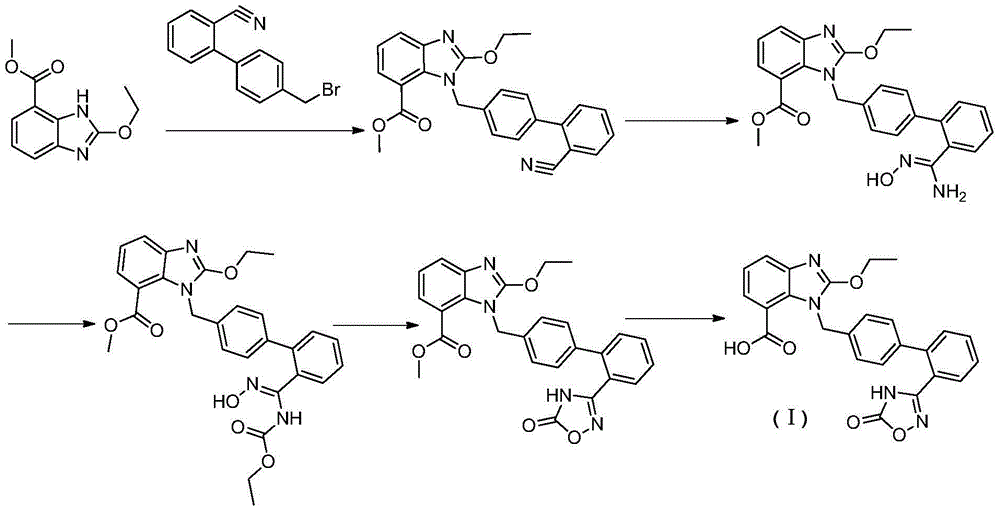
Preparation method of anti-hypertensive drug
The invention provides a preparation method of an anti-hypertensive drug. Specifically the invention provides a novel intermediate: 2-ethoxy-1-{[2'-(5-carbonyl-4,5-dihydro-1,2,4-oxadiazole-3-yl)biphenyl-4-yl]methyl}-1H-benzoimidazole-7-carboxylic acid (Azilsartan). The intermediate is represented by the formula A, wherein in the formula the X represents an H atom or a halogen atom. The Azilsartan can be rapidly and conveniently prepared through the prepared intermediate represented by the formula A. Moreover, the preparation method has the advantages of short reaction route, less by products, high total yield, and mild conditions, and is suitable for industrial production of Azilsartan.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
Method for preparing high-purity white carbon black from silicon-containing raw material
The invention discloses a method for preparing high-purity white carbon black from a silicon-containing raw material. The method specifically comprises the following steps: sufficiently stirring and uniformly mixing the silicon-containing raw material and sodium hydroxide to obtain a mixed raw material; carrying out oxidization roasting on the mixed raw material; then carrying out high-pressure leaching and filtering to obtain filtrate; diluting the filtrate with pure water to obtain high-purity sodium silicate; adding a chemical agent into the high-purity sodium silicate; and carrying out deposition, filtering and drying to, and calcining to obtain the high-purity white carbon black. The method disclosed by the invention comprises two processes of preparing the high-purity sodium silicateand preparing the white carbon black; sulfuric acid is not used in a whole process, so that the method is more environmentally friendly; the method has the advantages of novel route, great raw material selectivity and compact preparation technology; and the white carbon black prepared by the method has the advantages of extremely high purity, extremely stable quality and controllable impurity variety and content.
Owner:LONGZHOU WANHE TRADING CO LTD
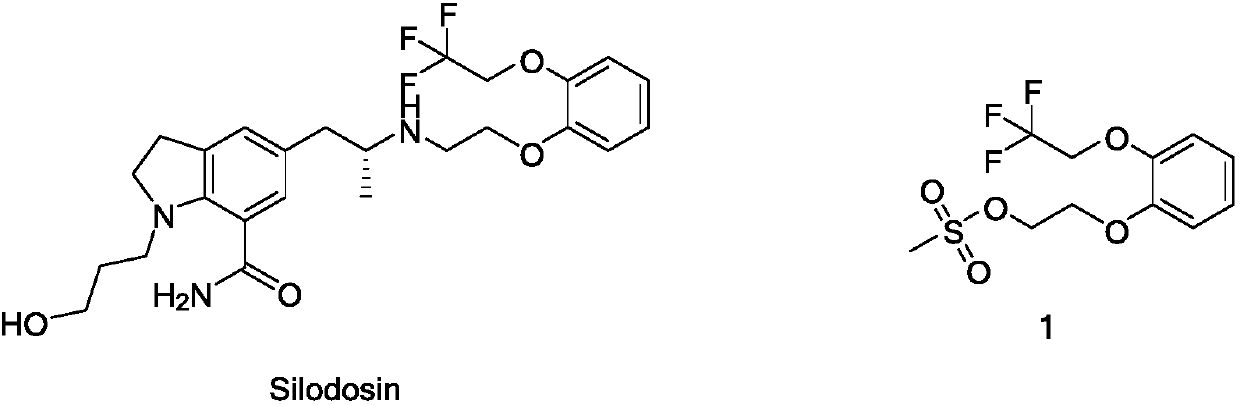
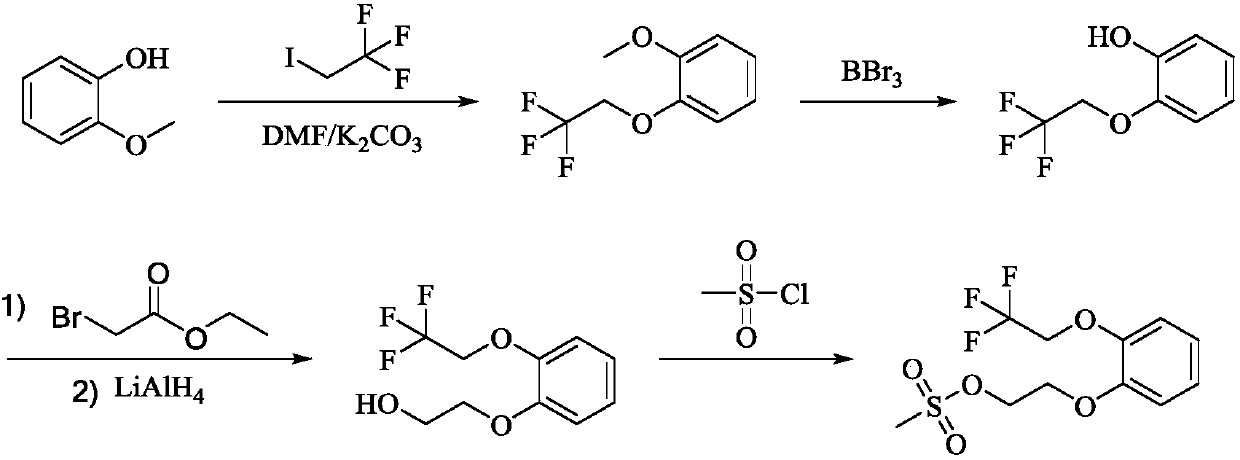
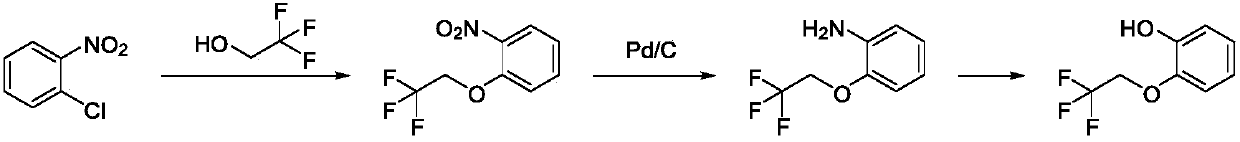
Preparation method of silodosin intermediate
ActiveCN109516933AHigh purityNew routeOrganic compound preparationSulfonic acid esters preparationChemical synthesisBenzaldehyde
The invention discloses a preparation method of a silodosin intermediate and relates to the technical field of chemical synthesis of drugs. The preparation method comprises the following steps: subjecting salicyaldehyde and ethylene carbonate to transesterification to obtain 2-(2-hydroxyethoxy)benzaldehyde; then, carrying out a Dakin oxidation reaction to obtain sodium 2-(2-hydroxyethoxy) phenol;then, subjecting sodium 2-(2-hydroxyethoxy) phenol and trifluoroethanol to an etherification reaction to obtain 2-[2-(2,2,2-trifluoroethyoxy)phenoxy]ethyl alcohol; and finally, subjecting 2-[2-(2,2,2-trifluoroethyoxy)phenoxy]ethyl alcohol and methanesulfonyl chloride to an esterfication reaction to obtain the silodosin intermediate 2-[2-(2,2,2-trifluoroethyoxy)phenoxy]ethyl methanesulfonate. The preparation method is novel and short in synthesis route, and a target product can be prepared by only four-step reaction. Both raw materials and reagents used for preparation are cheap, available andenvironment-friendly, all the reaction conditions are mild and controllable, the preparation method is convenient and simple in operation, and the prepared silodosin intermediate is high in purity andyield, suitable for industrial production and wide in prospect and industrial application value.
Owner:ANHUI QINGYUN PHARMA & CHEM
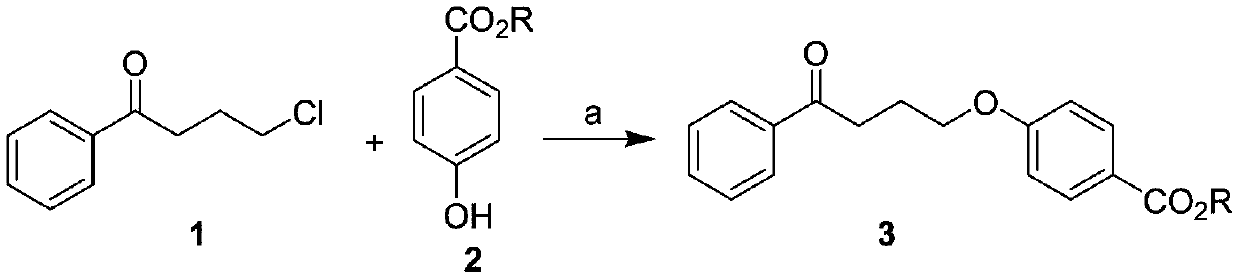
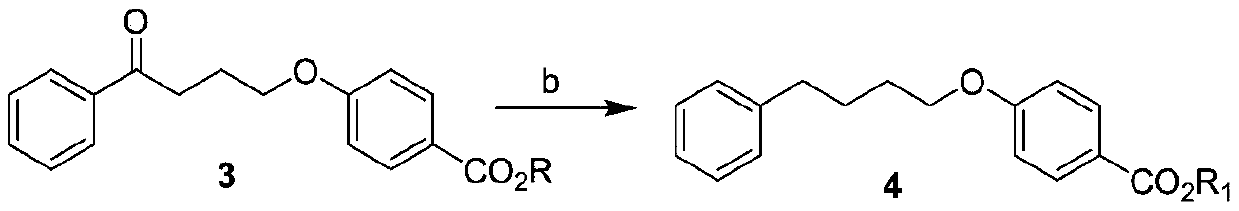
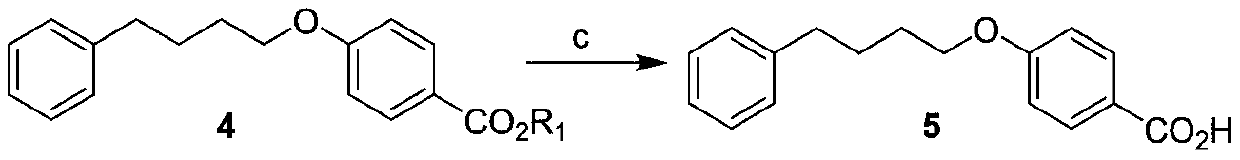
Preparation method of 4-(4-phenylbutoxy) benzoic acid
ActiveCN111592451ACheap and easy to getNew routeOrganic compound preparationCarboxylic acid esters preparationBenzoic acidChlorobenzene
The invention relates to a preparation method of 4-(4-phenylbutoxy) benzoic acid, which specifically comprises the following steps: a, performing a reaction on gamma-chlorobutanone with p-hydroxybenzoate under the catalysis of alkali to generate a compound 3; b, performing catalytic reduction on the compound 2 to generate a compound 4; and c, hydrolyzing the compound 4 to obtain the 4-(4-phenylbutoxy) benzoic acid. According to the preparation method of the 4-(4-phenylbutoxy) benzoic acid, the adopted raw materials are low in price and easy to obtain; the method has the advantages of short synthetic route, novel route, no pollution and high yield.
Owner:KUNSHAN RIKITA PHARMA
A kind of preparation method of 3-amino-2-hydroxyacetophenone
ActiveCN106831457BCheap and easy to getHigh purityOrganic compound preparationSulfonic acid preparationFries rearrangementHydrolysis
The invention discloses a new preparation method of a key intermediate, namely 3-amino-2-hydroxyphenylacetone, for preparation of Pranlukast. The new preparation method comprises the following main steps: taking 2-aminophenol-4-sulfonic acid as a starting raw material, and carrying out acylation, Fries rearrangement, hydrolysis and deprotection, so that 3-amino-2-hydroxyphenylacetone is obtained. Compared with the prior art, the new preparation method disclosed by the invention has the advantages that the used raw materials are cheap and easily available, technology can easily realize industrialization, and the obtained final product is high in purity; no danger technology is adopted, and equipment is simple; and route is novel, and synthesis route is short.
Owner:上海微巨实业有限公司
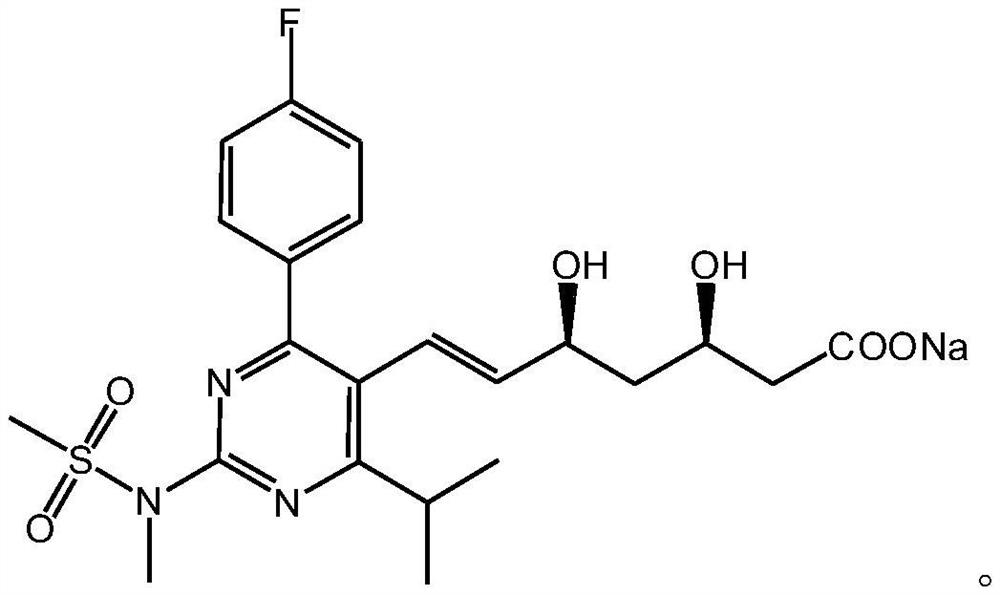
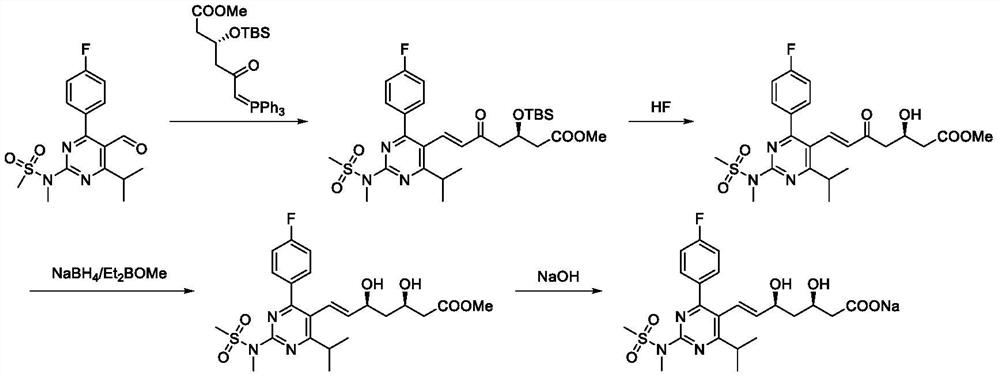
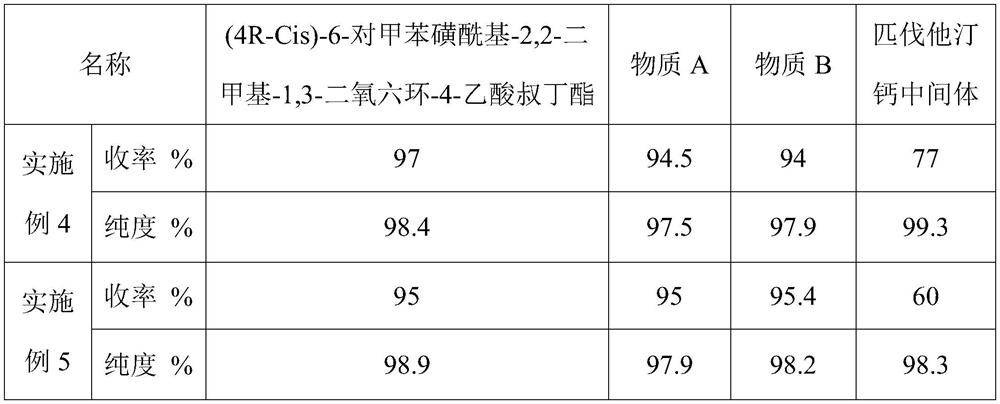
A kind of preparation method of rosuvastatin sodium
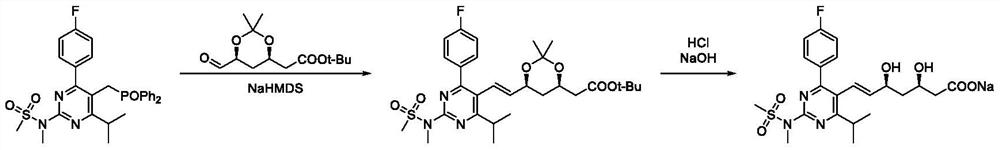
ActiveCN109574939BHigh purityHigh yieldOrganic chemistry methodsBulk chemical productionPtru catalystHydrolysis
The invention discloses a preparation method of rosuvastatin sodium, comprising the following steps: (4R-Cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4- The sulfonylation reaction of tert-butyl acetate and p-toluenesulfonyl chloride under the action of a catalyst gives (4R-Cis)-6-p-toluenesulfonyl-2,2-dimethyl-1,3-dioxane-4 ‑tert-butyl acetate; then react with trimercapto-s-triazine under the action of a basic catalyst to obtain substance A; then oxidize with oxidant to obtain substance B; then react with 4‑(4‑fluorophenyl)‑6‑isopropyl ‑2‑[(N‑Methyl‑N‑methylsulfonyl)amino]pyrimidine‑5‑formaldehyde is reacted under the catalysis of sodium hydride to obtain substance C; finally deprotected by hydrochloric acid, and sodium hydroxide is hydrolyzed into a salt to obtain rosuvastatin sodium.
Owner:ANHUI QINGYUN PHARMA & CHEM
A quinone-type chalcone compound with isopentenyl group, preparation method and anti-inflammatory activity
ActiveCN105085219BNovel structureNew routeOrganic active ingredientsNervous disorderQuinoneIsoamoenylin
The invention discloses a quinoid chalcone compound with an isopentenyl group at an A ring, and a preparation method and anti-inflammatory activity thereof. The compound has a structure as shown in a general formula (I) which is described in the specification. The preparation method comprises the following steps: (1) synthesizing 3,5-dihydroxy-2-acetyl-6,6-diisopentenylcyclohexyl-2,4-dienone; (2) synthesizing 3-hydroxy-5-methoxy-2-acetyl-6,6-diisopentenylcyclohexyl-2,4-dienone; (3) synthesizing a methoxy group protected quinoid chalcone compound with the isopentenyl group at the A ring; and (4) synthesizing the quinoid chalcone compound with the isopentenyl group at the A ring. The compound is simple to prepare and has obvious anti-inflammatory action.
Owner:稷冲(北京)医药有限公司
Preparation method of omarigliptin intermediate
ActiveCN107473988AStable in natureRaw materials are easy to getCarbamic acid derivatives preparationOrganic compound preparationAlderSynthesis methods
The invention discloses a novel synthesis method for preparing a chiral intermediate of omarigliptin. The method disclosed by the invention comprises the following steps: taking crotonyl chloride and (S)-4-benzyl-2-oxazolidone as starting raw materials and carrying out amidation, alder condensation, hydrolysis, Curtius rearrangement, Boc addition and ring opening to obtain the chiral intermediate (VI). The preparation method disclosed by the invention has the advantages of relatively short route, relatively low cost, easiness for obtaining the raw materials and relatively high yield so that the preparation method is suitable for industrial production. A formula is shown in the description.
Owner:ANHUI HAIKANG PHARMA
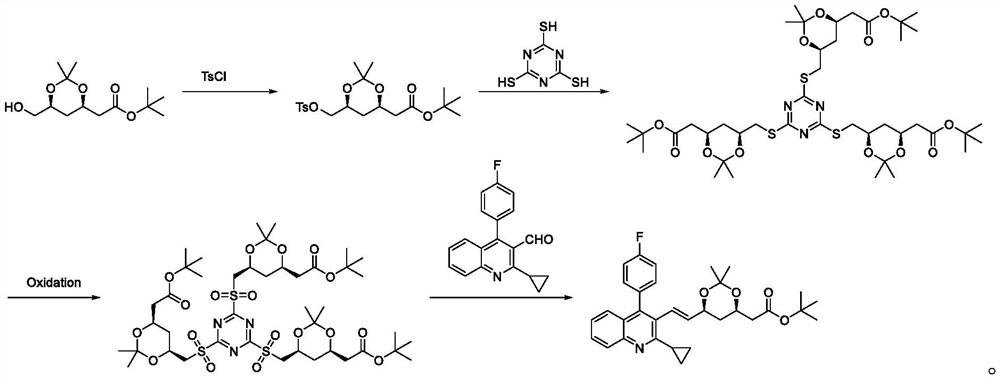
A kind of preparation method of pitavastatin calcium intermediate
The invention discloses a preparation method of pitavastatin calcium intermediate, comprising the following steps: (4R-Cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4 The sulfonylation reaction of tert-butyl acetate and p-toluenesulfonyl chloride under the action of a catalyst gives (4R-Cis)-6-p-toluenesulfonyl-2,2-dimethyl-1,3-dioxane- 4-tert-butyl acetate; then react with trimercapto-s-triazine under the action of a basic catalyst to obtain substance A; then oxidize to obtain substance B through the action of an oxidizing agent; finally react with 2-cyclopropyl-4-(4-fluorophenyl ) quinoline-3-formaldehyde is reacted under the catalysis of sodium hydride to obtain pitavastatin calcium intermediate. The raw material of the present invention is cheap and easy to obtain, good atom economy, green and environmental protection, mild and controllable reaction conditions, simple operation, simple purification treatment, suitable for industrial production, good stereoselectivity, high yield, and the prepared pitavastatin calcium intermediate Good purity.
Owner:ANHUI QINGYUN PHARMA & CHEM
The synthetic method of roxatidine intermediate 3-(1-piperidinylmethyl)phenol
The invention discloses a method for synthesizing Roxatidine intermediate 3-(1-piperidinyl methyl) phenol and relates to the technical field of organic synthesis. M-anisyl alcohol which is cheap and easy to obtain is used as a raw material and firstly reacts with sulfinyl chloride to produce m-methoxybenzyl chloride, then the m-methoxybenzyl chloride reacts with piperidine to produce 3-(1-piperidinyl methyl) anisole, and finally demethoxylation is performed in hydrobromic acid to obtain the target product 3-(1-piperidinyl methyl) phenol. The route is clear, the yield is high, the amount of produced waste water, gas and residues is small, the recycling of solvent can be realized, the preparation cost is reduced and the cost is less than half of the cost of the original route.
Owner:ANHUI HERYI CHEM
Preparation method for key intermediate of letrozole
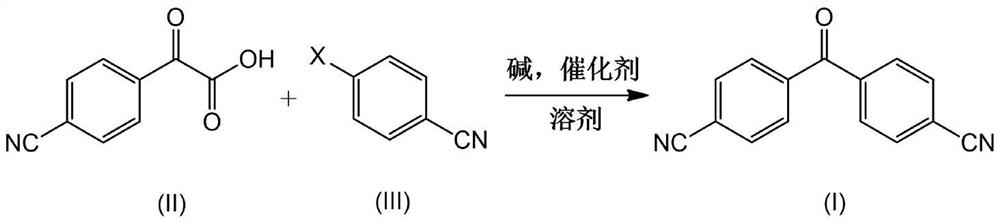
InactiveCN113072462AHigh purityReduce usageCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystBiochemical engineering
The invention provides a preparation method for a key intermediate (I) of letrozole. According to the method, 2-(4-cyanophenyl)formylformic acid (II) and 4-halogenated cyanophenyl (III) are used as raw materials and subjected to one-step reaction in the presence of alkali, a solvent and a catalyst so as to generate a target product. The preparation method is novel in route, simple and controllable in operation, high in reaction yield, economical and capable of ensuring that high-purity letrozole is obtained through subsequent preparation, and provides guarantees for industrial large-scale production of high-purity letrozole.
Owner:HANGZHOU ZHONGMEI HUADONG PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com