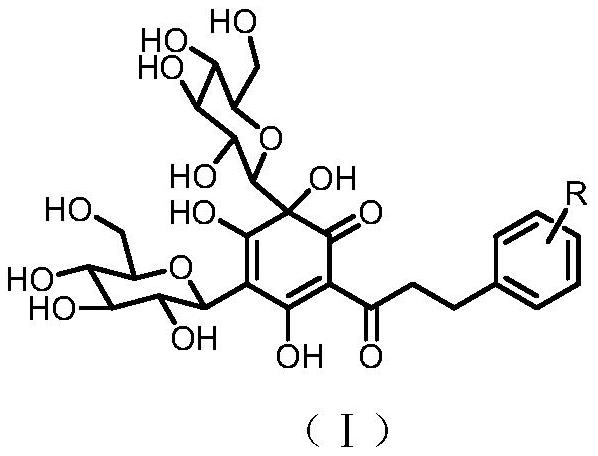
Quinoid dihydrochalcone dicarbonyl glycoside compound with glucose on A ring, preparation method and neuroprotective activity thereof
A technology of dihydrochalcone two-carbon glycoside compound and glucosyl dihydrochalcone two-carbon glycoside, which can be applied in the field of medicine and can solve the problems such as failure to achieve success
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The synthetic method of compound 1, comprises the following steps:
[0059] (1) Synthesis of 2,4,6-trihydroxydihydrochalcone
[0060] Take phloroglucinol (5.0g, 40mmol) and phenylpropionic acid (6.0g, 40mmol) in a 100mL double-necked bottle, add boron trifluoride in ether solution (26mL, 200mmol), under nitrogen protection, at 85°C Stir the reaction for 4h, lower to room temperature, pour the reaction solution into 10% sodium acetate aqueous solution (200mL), let it stand for 4h, extract three times with ethyl acetate, 200mL each time, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter In addition to the desiccant, concentrated under reduced pressure to obtain the crude product, silica gel column chromatography, the eluent is petroleum ether / ethyl acetate = 5:1, 5.36g of 2,4,6-trihydroxydihydrochalcone was obtained, and the yield was 52%; the process takes place as follows:
[0061]
[0062] The structural characterizat...
Embodiment 2
[0071] The synthetic method of compound 2, comprises the following steps:
[0072] (1) Synthesis of 2,4,6,13-tetrahydroxydihydrochalcone
[0073] Take phloroglucinol (5.0g, 40mmol) and p-hydroxyphenylpropionic acid (6.24g, 40mmol) in a 100mL two-necked bottle, add boron trifluoride in ether solution (26mL, 200mmol), under air protection, 85°C Stir the reaction for 4 h under the conditions, lower to room temperature, pour the reaction solution into 10% sodium acetate aqueous solution (200 mL), let it stand for 4 h, extract three times with ethyl acetate, 200 mL each time, combine the organic phases, wash with saturated brine, and dry over anhydrous sodium sulfate , filtered off the desiccant, and concentrated under reduced pressure to obtain a crude product, which was subjected to silica gel column chromatography, and the eluent was petroleum ether / ethyl acetate=3:1 to obtain 6.58 g of 2,4,6,13-tetrahydroxydihydrochalcone , the productive rate is 60%; The process takes place i...
Embodiment 3
[0085] The synthetic method of compound 3 comprises the following steps:
[0086] (1) Synthesis of 2,4,6-trihydroxy-13-methoxydihydrochalcone
[0087] Take phloroglucinol (5.0g, 40mmol) and p-methoxyphenylpropionic acid (7.2g, 40mmol) in a 100mL double-necked bottle, add boron trifluoride ether solution (26mL, 200mmol), under nitrogen protection, Stir the reaction at 85°C for 4 hours, cool down to room temperature, pour the reaction solution into 10% sodium acetate aqueous solution (200mL), let stand for 4h, extract three times with ethyl acetate, 200mL each time, combine the organic phases, wash with saturated brine, anhydrous sulfuric acid Drying over sodium, filtering off the desiccant, and concentrating under reduced pressure to obtain the crude product, silica gel column chromatography, the eluent is petroleum ether / ethyl acetate = 4:1, to obtain 2,4,6,13-tetrahydroxydihydrochalcone 7.49g, productive rate is 65%; This process takes place following reaction:
[0088] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com