Preparation method of larotinib intermediate, and intermediate compound
A larotrectinib and compound technology, which is applied in the preparation of larotrectinib intermediates and the field of intermediate compounds, can solve the problems of unsuitability for industrial production, high price and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
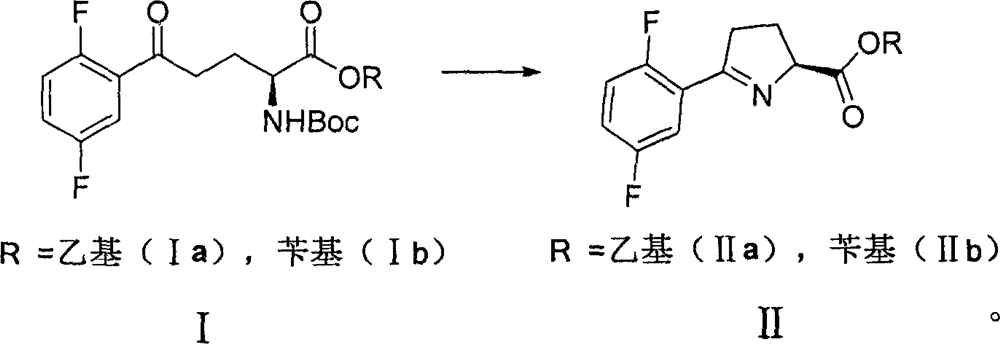
[0039] Example 1 Synthesis of intermediate I (Ia, Ib)
[0040]
[0041]Synthesis of Ia: In a 500mL three-necked flask, add 19.3g (0.1mol) of p-2,5-difluorobromobenzene and 160ml of tetrahydrofuran, cool to 0 degrees, start to drop 60ml of isopropylmagnesium chloride (2M THF solution, 0.12 mol), after the dropwise addition, continue to react for 2h; then dropwise add 25.7g (0.1mol) tetrahydrofuran 80ml solution of N-tert-butoxycarbonyl-L-pyroglutamic acid ethyl ester, after the dropwise addition, react at 0 degree for 4h, TLC showed the reaction was complete. Add 50ml of 2N hydrochloric acid solution dropwise to the reaction mixture, stir for 20 minutes, separate the layers, extract the aqueous phase twice with 120ml of methyl tert-butyl ether, combine the organic phases, wash with 60ml of saturated saline, and dry over anhydrous sodium sulfate , filtered, concentrated under reduced pressure, and separated by silica gel column chromatography to obtain 35.2 g of oily substan...
Embodiment 2
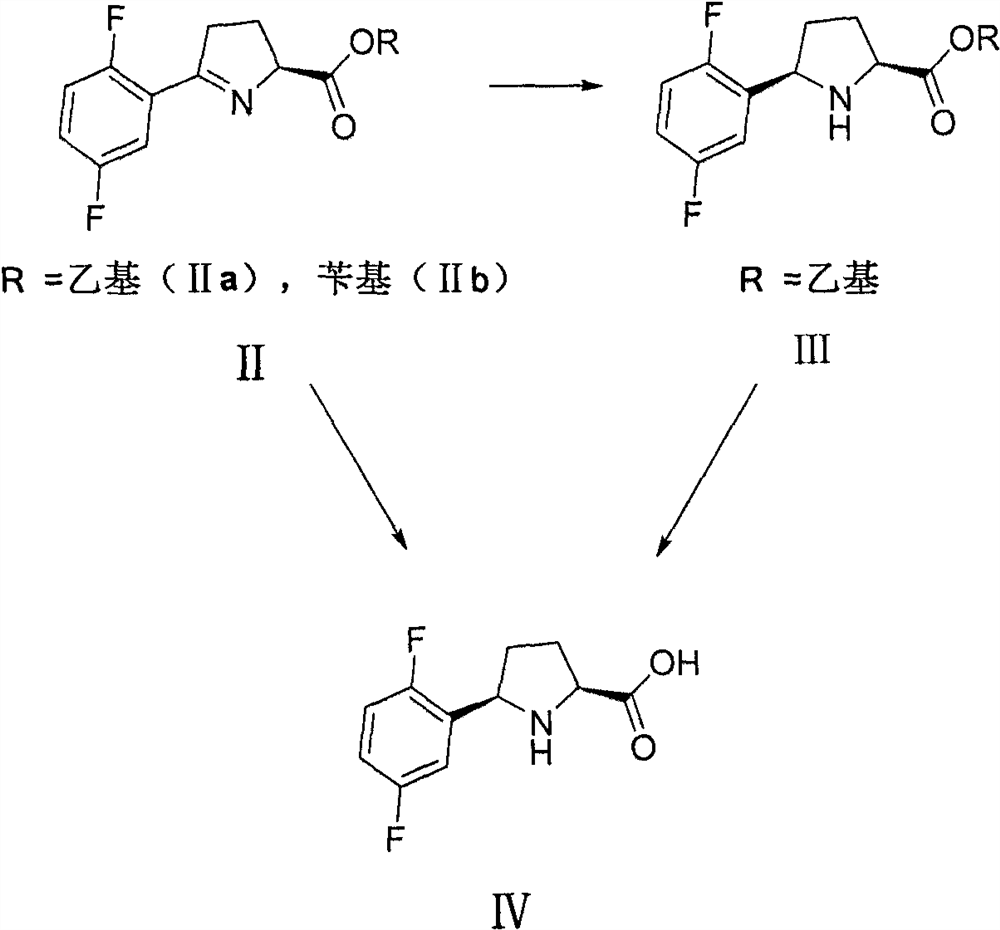
[0047] Example 2 Synthesis of Intermediate II (IIa, IIb)
[0048]
[0049] Synthesis of IIa: In a 250ml flask, 18.6g (0.05mol) of compound Ia and 15ml of dichloromethane were added, cooled to 0°C, and 15ml (0.2mol) of trifluoroacetic acid was added dropwise. After the dropwise addition, the reaction was stirred at 0°C, and the reaction was tracked by TLC until the raw material of compound Ia disappeared. Then use 10% sodium hydroxide solution to adjust the pH value to 8, add 80ml of dichloromethane to separate layers, extract the aqueous layer twice with dichloromethane, 50ml each time, combine the organic phases, dry over anhydrous sodium sulfate, and concentrate under reduced pressure , separated by silica gel column chromatography to obtain IIa11.6g with a yield of 92% and a purity of 98.3%.
[0050] 1 HNMR (400Hz, CDCl 3 )δ: 7.72-7.81 (m, 1H), 7.05-7.17 (m, 2H), 4.85-4.95 (m, 1H), 4.25 (q, 2H), 3.14-3.26 (m, 1H), 3.00-3.12 ( m, 1H), 2.31-2.45(m, 1H), 2.15-2.32(m, 1H...
Embodiment 3
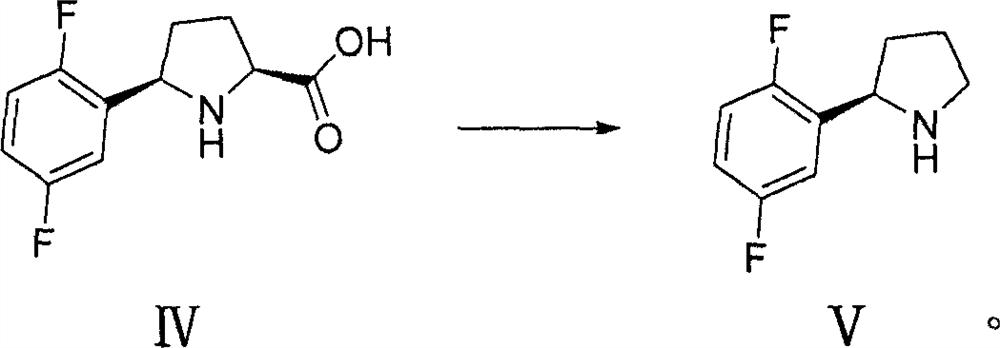
[0055] Example 3 Synthesis of Intermediate IV
[0056]
[0057] method one:
[0058] Add 10 g (0.04 mol) of compound IIa, 1 g of 10% palladium on carbon and 150 ml of methanol in a hydrogenation reactor to hydrogenate to 5 atmospheres, react at room temperature for 5 h, follow the reaction by TLC until the raw material disappears. It was filtered, concentrated under reduced pressure, and separated by silica gel column chromatography to obtain 9.7 g of III with a yield of 95% and a purity of 99%.
[0059] Alternatively prepare III by:
[0060] In a 250ml flask, 5.0g (0.02mol) of compound IIa, 50ml of methanol and 20ml of acetic acid were added, cooled to -40°C, and 1.52g (0.04mol) of sodium borohydride was added in batches. After the dropwise addition was completed, stirred for 1 h, raised to 0°C and stirred for 2 h, and followed the reaction by TLC until the raw material of compound IIa disappeared. Then it was quenched with 10% sodium carbonate solution, concentrated un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com