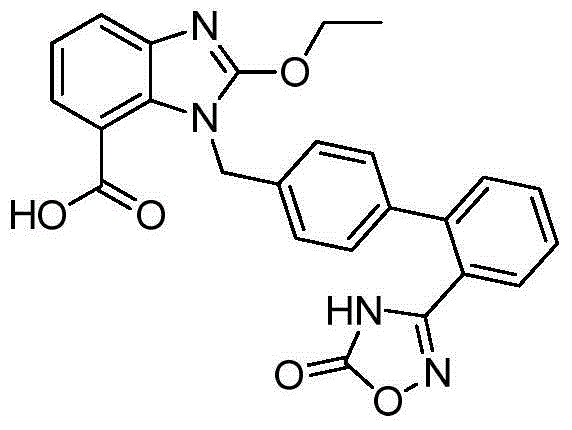
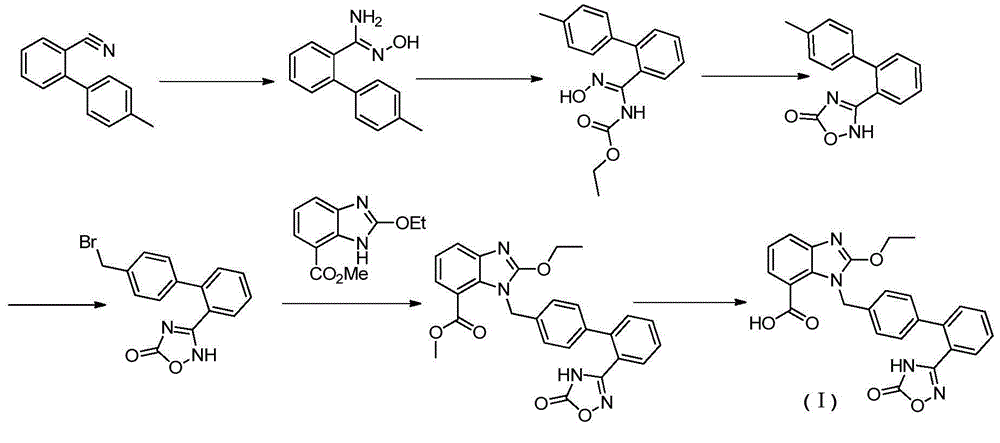
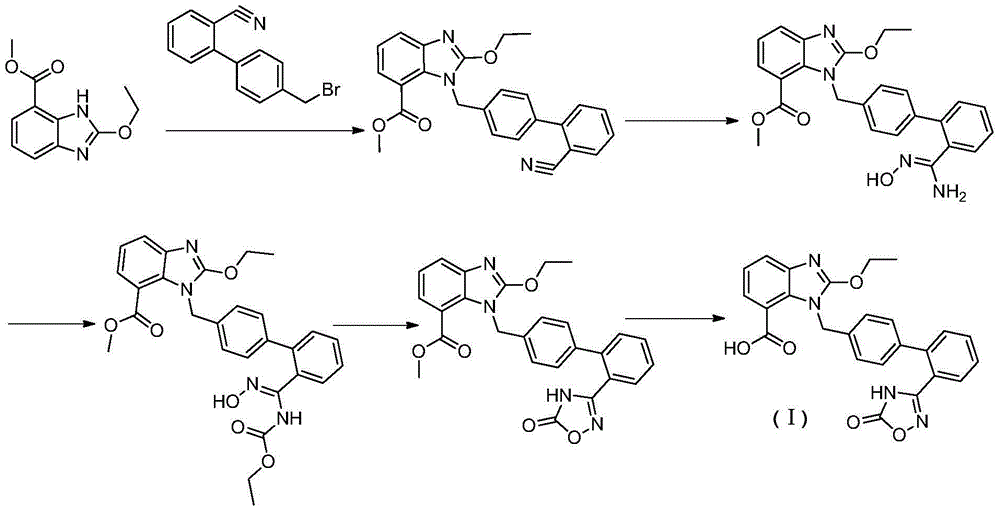
Preparation method of anti-hypertensive drug
A compound, inert solvent technology, applied in the field of chemical pharmacy, can solve the problems of less by-products, high raw material price, danger, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0099] The preparation method of described formula (IV) compound comprises steps:
[0100] (2) In an inert solvent, the compound of formula (III) is reacted with a halogenating reagent to obtain a compound of formula A;
[0101]
[0102] In the formula, X is a halogen.
[0103] Preferably, in the step (2), the halogenation reagent is selected from the group consisting of chlorine, NCS, thionyl chloride, phosphorus trichloride, phosphorus oxychloride, phosphorus pentachloride, trichloroisocyanate Uric acid, or combinations thereof; preferably NCS, trichloroisocyanuric acid, or combinations thereof.
[0104] In the step (2), the inert solvent is not particularly limited, and the preferred inert solvent includes (but not limited to) a solvent selected from the following group: dichloromethane, tetrahydrofuran, toluene, xylene, di Hexane, n-heptane, n-hexane, or combinations thereof; preferably dichloromethane.
[0105] Preferably, in the step (2), the reaction temperature i...
Embodiment 1
[0179] (1) Preparation of compound (III):
[0180] Add hydroxylamine hydrochloride solution (10.63g, 152.87mmol, water 50ml) into 20% aqueous sodium hydroxide solution (25mL) at room temperature, and stir at this temperature for 15min, then add 2-formyl-4'-methyl Biphenyl (II) (20.0g, 101.92mmol), and continued to react at this temperature for 2h, and TLC detected that the reaction was complete. After suction filtration, the filter cake was washed three times with water, and dried to obtain 21.1 g of compound (III), with a yield of 98%.
[0181] (2) Preparation of compound (IV):
[0182]Add compound (III) (21.0g, 99.41mmol), DMF (10mL) and DCM (500mL) to a 1L round bottom flask, cool to 0°C, slowly add NCS (14.6g, 109.35mmol), after the addition is complete, place at room temperature After stirring for 2 h, TLC detected that the reaction was complete. Pour the reaction solution into water (300mL), stir for 15min, take the organic phase, wash with water (200mL) and saturated...
Embodiment 2
[0193] (1) Preparation of compound (III):
[0194] Add hydroxylamine hydrochloride solution (10.63g, 152.87mmol, water 50ml) into 20% aqueous sodium hydroxide solution (25mL) at room temperature, and stir at this temperature for 15min, then add 2-formyl-4'-methyl Biphenyl (II) (20.0g, 101.92mmol), and continued to react at this temperature for 2h, and TLC detected that the reaction was complete. After suction filtration, the filter cake was washed three times with water, and dried to obtain 20.9 g of compound (III), with a yield of 97%.
[0195] (2) Preparation of compound (IV):
[0196] Add compound (III) (20.5g, 97.04mmol), DMF (10mL) and DCM (500mL) to a 1L round bottom flask, cool to 0°C, slowly add NCS (14.25g, 106.74mmol), after the addition is complete, place at room temperature After stirring for 2 h, TLC detected that the reaction was complete. Pour the reaction solution into water (300mL), stir for 15min, take the organic phase, wash with water (200mL) and saturat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com