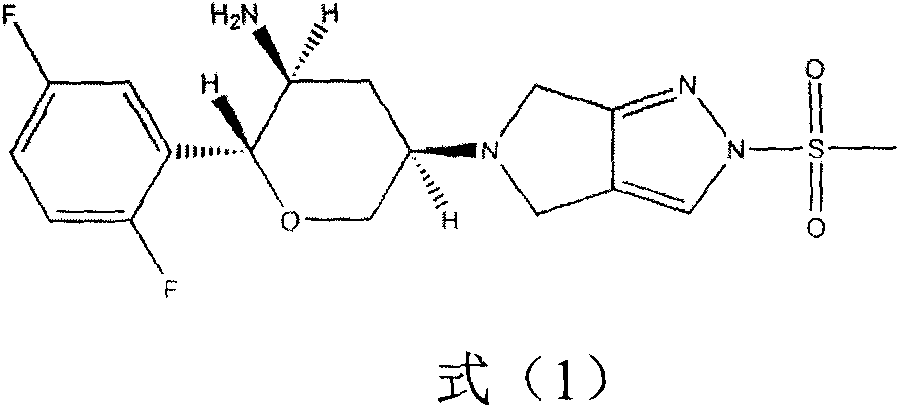
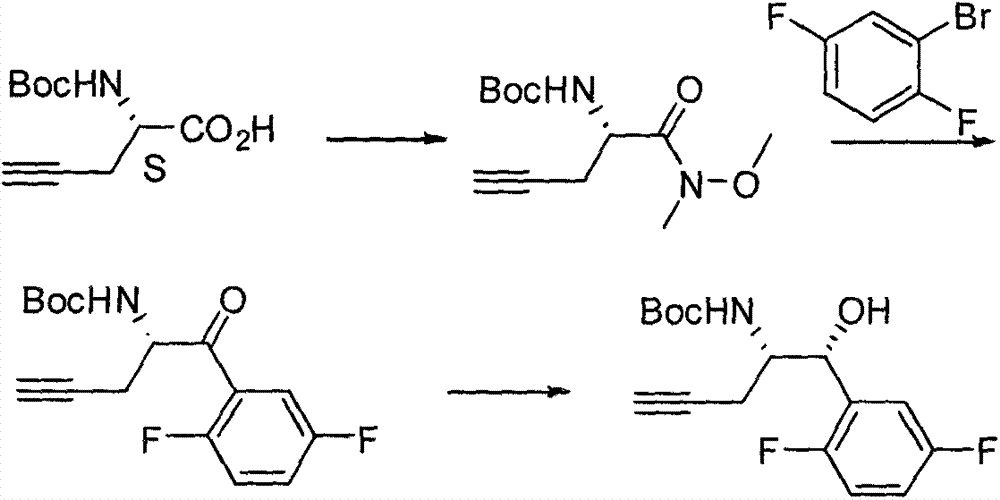
Preparation method of omarigliptin intermediate
A technology of intermediates and chiral intermediates, which is applied in the field of preparation of alogliptin intermediates, can solve the problems of unavailable raw materials, high costs, and low yields, and achieve novel routes, stable properties, and high yields Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis of embodiment 1 intermediate (I)
[0035]
[0036] Mix 12g (0.123mol) propargyl urea and 42.8ml (0.308mol) triethylamine, add 200ml of anhydrous THF, under the protection of nitrogen, lower the temperature to -25°C, slowly add 16.6ml (0.135mol) ) pivaloyl chloride, the dropwise addition was completed, and stirred for 2 hours. Then add 5.74g (0.135mol) anhydrous lithium chloride and 20g (0.123mol) (S)-4-benzyl-2-oxazolidinone successively, then slowly warming up to room temperature, stirring overnight, TLC shows that the raw material has disappeared, THF was removed under reduced pressure, ethyl acetate was added, washed twice with water, dried, concentrated, and silica gel column chromatography gave intermediate (I), 28.4g, yield: 90%, [M+H + ]: 258.1.
Embodiment 2
[0037] The synthesis of embodiment 2 intermediate (II)
[0038]
[0039] Method 1: replace the nitrogen reaction bottle, add 25.7g (0.1mol) of intermediate (I) and 163ml of anhydrous dichloromethane, lower the temperature to -5°C, and slowly add 32.9g (0.12mol) of trifluoroform Dibutylboron sulfonate, stirred for 10 minutes after dropping. Then 21 ml (0.15 mol) of triethylamine were added dropwise. After the dropwise addition was completed, the temperature was lowered to -70° C., and a solution of 15 g (0.105 mol) of 2,5-difluorobenzaldehyde in 50 ml of dichloromethane was slowly added dropwise. The temperature rose to -10°C over 1 hour and stirred for 1 hour. TLC shows that the reaction is complete, adding 102ml of potassium phosphate buffer successively, 53ml of methyl alcohol and 53ml of 35% hydrogen peroxide, separating the organic layer, washing with saturated sodium bicarbonate, washing with sodium thiosulfate, drying, and separating the intermediate (II) by silica ...
Embodiment 3
[0041] The synthesis of embodiment 3 intermediate (III)
[0042]
[0043] Into the reaction flask, 20 g (0.05 mol) of intermediate (II) 13.7 g (0.053 mmol), 226 ml of tetrahydrofuran and 57 ml of water were added. The temperature was cooled to 0 °C. 21.3ml (0.185mol) of 30% hydrogen peroxide and 80ml (0.08mol) of 1M lithium hydroxide were added dropwise in sequence. After the dropwise addition was completed, the reaction was stirred for 2 hours. TLC showed that the reaction was complete, and 142 ml of aqueous sodium sulfite (25 g of sodium sulfite) was added, extracted once with dichloromethane, and the pH value of the aqueous layer was adjusted to 2-3 with concentrated sulfuric acid. Ethyl acetate was extracted three times, the organic layers were combined, dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and separated by silica gel column chromatography to obtain 11.4 g of intermediate (III), with a yield of 95%, [M+H + ]: 241.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com